
a)
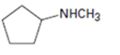
Interpretation:
The major product expected when the
Concept introduction:
Hofmann’s rule is applicable to elimination reactions involving positively charged and bulky leaving groups. According to this rule, when two possibilities exist in an elimination reaction, the hydrogen is eliminated from the carbon with more number of hydrogen atoms so that a less substituted
b)
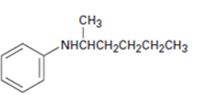
Interpretation:
The major product expected when the amine shown undergoes Hofmann elimination is to be given.
Concept introduction:
Hofmann’s rule is applicable to elimination reactions involving positively charged and bulky leaving groups. According to this rule, when two possibilities exist in an elimination reaction, the hydrogen is eliminated from the carbon with more number of hydrogen atoms so that a less substituted alkene will be the major product.
c)
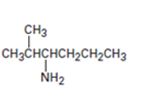
Interpretation:
The major product expected when the amine shown undergoes Hofmann elimination is to be given.
Concept introduction:
Hofmann’s rule is applicable to elimination reactions involving positively charged and bulky leaving groups. According to this rule, when two possibilities exist in an elimination reaction, the hydrogen is eliminated from the carbon with more number of hydrogen atoms so that a less substituted alkene will be the major product.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- Please see photoarrow_forward=Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

