
Interpretation:
Using benzene and any other necessary
Concept introduction:
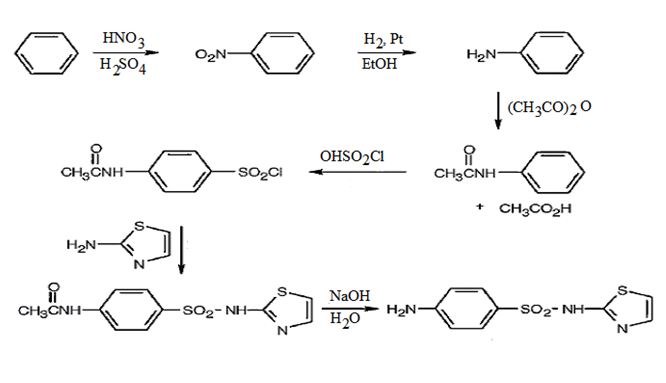
The steps involved in the synthesis of sulfathiazole from benzene are i) Nitration of benzene ii) Reduction of nitrobenzene iii) Acetylation of the amino group iv) Chlorosulfonation of the anilide v) Treatment with 2-aminothiazole vi) Hydrolysis of the anilide to an amine.
To Show:
How to synthesize sulfathiazole starting from benzene and using any other necessary amines.
Answer:
The synthesis of sulfathiazole starting from benzene and using any other necessary amines is given below.
Explanation:
Nitration of benzene with Conc. HNO3 and Conc. H2SO4 yields nitrobenzene which on reduction with H2/Pt in ethanol gives aniline. Acetylation of aniline with acetic anhydride produces acetanilide. Chlorosulfonation of acetanilide leads to the formation of a p-chlorosulfonate as anilide group is orthp and para directing. Treatment of chlorosulfonate with 2-aminothiazole yields N-acetylsulfothiazole. Hydrolysis of N-acetylsulfothiazole yields sulfathiazole.
Conclusion:
The synthesis of sulfathiazole starting from benzene and using 2-aminothiazole is given below.

Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- In the presence of a trace of acid, d@hydroxyvaleric acid forms a cyclic ester (lactone).HO¬CH2CH2CH2CH2¬COOHd@hydroxyvaleric acid(a) Give the structure of the lactone, called d@valerolactone.(b) Propose a mechanism for the formation of d@valerolactone.arrow_forwardBenzocaine (ethyl p-amino benzoate) is a local anesthetic compound. Design its synthesis using the necessary reagents from toluene.arrow_forwardThe following three derivatives of succinimide are anticonvulsants that have found use in the treatment of epilepsy, particularly petit mal seizures. Q. Show how this same synthetic strategy can be used to prepare ethosuximide and methsuximide.arrow_forward
- Propose a synthesis for propoxyphene from 1-phenyl-1-propanone and any other necessary reagents.arrow_forwardPropose a synthesis for the following compund starting from phenylhydrazine and the corresponding ketone.arrow_forwardPropose a synthesis pathway for the production of the following compounds (d) to (e) from the given starting materials plus necessary organic and inorganic reagents.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

