
Concept explainers
(a)
Interpretation:
Thecomplete electron dot structure of naproxen needs to be determined by adding the lone pair and hydrogen atoms to the line drawing.
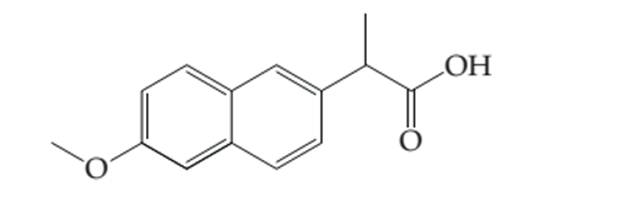
Concept introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom.
Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(b)
Interpretation:
Thecurve arrows in the electron dot structure of naproxen needs to be determined to generate the resonance structures.
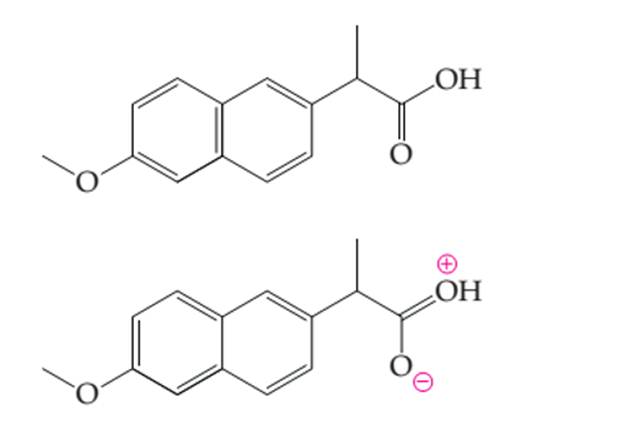
Concept introduction:
Organic compounds are the compounds which are mainly composed C and H atoms. The branch of chemistry that deals with preparation, reactions, and properties of organic compounds. The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
CHEMISTRY-TEXT
- Using just a periodic table (not a table of electronegativities), decide which of these is likely to be the most polar bond. Explain your answer! (a) C-F (b) S-F (c) Si-F (d) O-Farrow_forwardWhich of the following is NOT a valid resonance structure for anionic compound X? द् (B) क्ष X (C) & (D)arrow_forwardanswer all sub partsarrow_forward
- Which compounds have nonpolar covalent bonds, which have polar covalent bonds, and which have ions? (a) LiF (b) CH3F (c) MgCl2 (d) HClarrow_forwardAnswer true or false. (a) According to the Lewis model of bonding, atoms bond together in such a way that each atom par- ticipating in the bond acquires an outer-shell electron configuration matching that of the noble gas nearest to it in atomic number. (b) Atoms that lose electrons to achieve a filled valence shell become cations and form ionic bonds with anions. (c) Atoms that gain electrons to achieve filled valence shells become anions and form ionic bonds with cations. (d) Atoms that share electrons to achieve filled valence shells form covalent bonds. (e) Ionic bonds tend to form between elements on the left side of the Periodic Table, and covalent bonds tend to form between elements on the right side of the Periodic Table. (f) Ionic bonds tend to form between a metal and a nonmetal. (g) When two nonmetals combine, the bond between them is usually covalent. (h) Electronegativity is a measure of an atom’s attrac- tion for the electrons it shares in a chemical bond with another…arrow_forwardIdentify the electron geometry about each charged atom. Where appropriate, indicate the molecular geometry and approximate bond angle as well. (a) (b) (c) (d) CH3 (e) (f) O `NH H3CN-CH3 CH3arrow_forward
- Chemistry (a) Write three more resonance structures for each of compounds 1 and 2. (b) In each of compounds 1 and 2, determine which resonance structure contributes the most and explain your answer. (c) Are the 3/4 structures resonance structures or different compounds? Same question for 5/6 structures. Explain your answers.arrow_forwardFormic acid has the chemical formula HCOOH. It is a colorlessliquid that has a density of 1.220 g/mL. (a) The carbonatom in formic acid is bound to one H and both O’s. Drawthe Lewis structure for formic acid, showing resonance ifpresent. (b) Formic acid can react with NaOH in aqueoussolution to produce the formate ion, HCOO-. Write thebalanced chemical equation for this reaction. (c) Draw theLewis structure of the formate ion, showing resonance ifpresent. (d) How many milliliters of a 0.100 M solution ofNaOH would it take to completely react with 0.785 mL offormic acid?arrow_forwardDraw Lewis structures and any contributing resonance structures for each structure below. Use the proper type of arrow to indicate that they are resonance structures. In which structure is resonance more important? (a) H2SO3 (b) (HSO3)^1- (c) (SO3)^2-arrow_forward
- Classify the bond formed between each pair of atoms as covalent, polar covalent, or ionic.(a) Sr and F (b) N and Cl (c) N and Oarrow_forwardWhich statements are true about electronegativity? (a) Electronegativity increases from left to right in a period of the Periodic Table. (b) Electronegativity increases from top to bottom in a column of the Periodic Table . (c) Hydrogen, the element with the lowest atomic number, has the smallest electronegativity. (d) The higher the atomic number of an element, the greater its electronegativity.arrow_forwardConsider the theoretical molecule KrCl3‾. (a) Draw a valid Lewis structure for KrCl3‾. Show all lone pairs and use lines for bonds. Label all non-zeroformal charges on individual atoms and show the overall charge, if it exists, using square brackets.(b) What would you expect to be the molecular geometry for this ion? Fully explain your thought process,including all details about how successive lone pairs should be positioned within this electron geometry.(c) Draw this ion in 3-D, using hashed and wedged bonds as appropriate. Do not worry about labeling the overall or formal charge.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





