
Concept explainers
Interpretation:
The optical activity for the given compounds is to be deduced and open-chain structure for the aldaric acid is to be written. Fischer projection formulas for D-threose and its nitric acid oxidation product is to be written and name of aldaric acids produced from D-erythrose and D-threose is to be deduced.
The structural formulas of the given compounds are to be represented and the true or false nature of the statements based on the optical activity for the given compounds, is to be deduced.
Concept introduction:
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two
舧 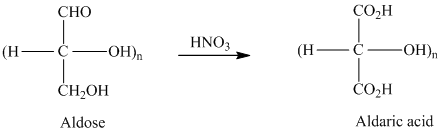
The molecules that are nonsuperimposable or not identical with their mirror images are known as chiral molecules.
舧 A pair of two mirror images that are nonidentical is known as enantiomers, which are optically active.
舧 The stereoisomers that are nonsuperimposable on each other and not mirror images of each other are known as diastereomers.
舧 The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds, but they are optically inactive.
舧 Compounds that have a plane of symmetry tend to exist in meso forms. A meso form arises when the two stereoisomers produce superimposable images, and hence, compounds having meso forms are optically inactive.
舧 When the order of progression from the group of highest priority to that of the next highest priority is clockwise, it is said to be the
舧 When the order of progression from the group of highest priority to that of the next highest priority is anticlockwise, then it is said to be the
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
- Don't used Ai solutionarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this kindly solve all parts and draw it not just word explanations!!arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


