
Concept explainers
Interpretation:
The structure of the disaccharide trehalose based on the given information’s provided is to be identified.
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, while axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
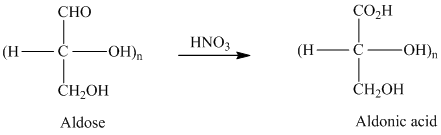
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
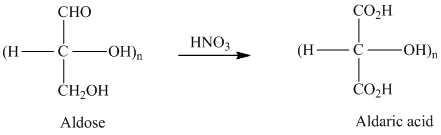
舧 Monosaccharides containing six carbon atoms and an aldehyde group are called aldohexoses.
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 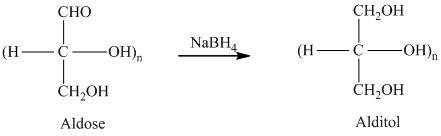
舧 Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 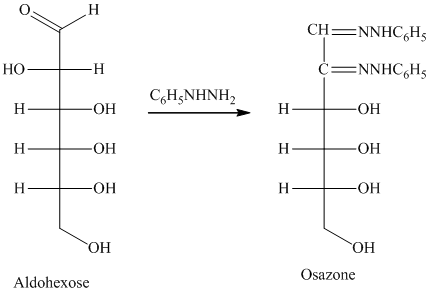
舧 Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
舧. Bromine water is an effective reagent that selectively oxidizes the
舧 Trehalose is a disaccharide found in yeasts, fungi, sea urchins, algae and insects. Its molecular formula is
舧 Sugars that react positively with Tollen’s reagent or Benedict’s solution are called reducing sugars, while those that do not react with Tollen’s reagent are called nonreducing sugars.
舧 The aldehyde group of an aldose reacts with three moles of phenylhydrazine to produce phenylosazone at
舧 Mutarotation is the change in values of specific rotation of each anomer while attaining equilibrium in an aqueous solution.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





