
Concept explainers
Interpretation:
The stereochemistry of
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, whereas axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The stability of the atom can be estimated at the values of the anomeric effect of the
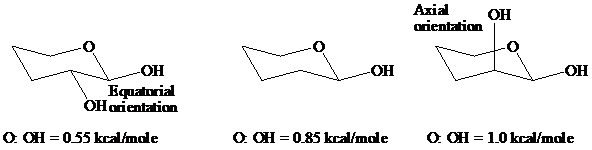
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?arrow_forwardThe most stable conformation of the pyranose ring of most D-aldohexoses places the largest group, CH2OH, in the equatorial position. An exception to this is the aldohexose D-idose. Draw the two possible chair conformations of either the a or ß anomer of D-idose. Explain why the more stable conformation has the CH2OH group in the axial position.arrow_forwardIf the following compound is saponified with sodium hydroxide, the products are: O || CH3(CH2) 14CH2-C-O-CH₂CH3 an ester and an alcohol an alcohol and a salt an acid and a salt an acid and an alcohol The purpose of the acid catalyst in the hydrolysis of an amide is: to enhance the nucleophilicity of the water molecule to enhance the electrophilicity of the amide carbonyl carbon to enhance the electrophilicity of the water molecule to shift the equilibrium of the reaction Which of the following compounds has the highest boiling point? CH3CH3 CH3CH2OH CH3-0-CH3 CH3COOHarrow_forward
- A graduate student was studying enzymatic reductions of cyclohexanones when she encountered some interesting chemistry. When she used an enzyme and NADPH to reduce the following ketone, she was surprised to find that the product was optically active. She carefully repurified the product so that no enzyme, NADPH, or other contaminants were present. Still, the product was optically active. Does the product have any asymmetric carbon atoms or other stereocenters?arrow_forwardLook up the structure of lisdexamfetamine (Vyvanse), a drug used in the treatment of attention deficit hyperactivity disorder (ADHD). Redraw it and identify all the functional groups present. What is known about itstherapeutic properties?arrow_forwardBetamethasone is a synthetic anti-inammatory steroid used as a topical cream for itching. Betamethasone is derived from cortisol, with the following structural additions: a C=C between C1 and C2, a uorine at C9, and a methyl group at C16. The configuration at C9 is R, and the configuration at C16 is S. Draw the structure of betamethasone.arrow_forward
- Tropone is an unusually basic carbonyl (C=O) compound. When it is treated with one equivalent of the strong acid HBF4, it forms A, C7H7OBF4. Draw the strucutre of A as its most stable resonance structure.arrow_forward(b) Cortisone OH H2C CH -HO- H3C Oalcohol O carbon-carbon double bond Oester Oaldehyde Ocarbon-carbon triple bond Oether O amide Ocarboxylate anion Oketone Oamine O carboxylic acid O thiol (c) Prostaglandin PGE2 COOH Но OH Oalcohol O carbon-carbon double bond Oester Oaldehyde Ocarbon-carbon triple bond Oether Oketone O amide O carboxylate anion Oamine Ocarboxylic acid O thiolarrow_forwardDenote how many nucleophilic centers are present in the following molecular structure (in case of polar bonds show clearly on the structure the dipoles õ+ and d-). :o: сноarrow_forward
- Which of the following functional group (FG) will produce a ketone upon oxidation with K2CrO7? (a) RX (b) 10OH (b) 20OH (c) 30OHarrow_forwardy-Butyrolactone (C4H6O2, GBL) is a biologically inactive compound that is converted to the biologically active recreational drug GHB (Section 19.5) by a lactonase enzyme in the body. Since y-butyrolactone is more fat soluble than GHB, it is more readily absorbed by tissues and thus produces a faster onset of physiological symptoms. y-Butyrolactone shows an absorption in its IR spectrum at 1770 cm-1 and the following 1H NMR spectral data: 2.28 (multiplet, 2 H), 2.48 (triplet, 2 H), and 4.35 (triplet, 2 H) ppm. What is the structure of y-butyrolactone?arrow_forwarda-Linolenic acid ((9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid; ALA)) is a poly-unsaturated, w-3 fatty acid with 18 carbons found in many food sources. Since humans cannot synthesize fats with double bonds past carbon #9, it is "essential" and must be obtained from the diet. Hope you like salmon! If a serving of salmon contains 500.0 mg of ALA and all of it is completely oxidized via B-oxidation and the TCA, how many grams of water at room temperature can be brought to a boil using that energy? (Specific heat of water: 4.184 J/mol; heat of vaporization of water: 40.65 kJ/mol; molar mass of ATP: 507.2 g/mol; DG° of ATP hydrolysis = -30.3 kJ/mol)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


