
Concept explainers
Interpretation:
The other product yield when compounds
Concept introduction:
舧 A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen-oxygen atom ratio of 2:1
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two
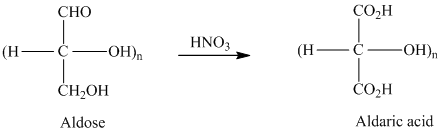
舧 The molecules that are nonsuperimposable or not identical with their mirror images are known as chiral molecules.
舧 A pair of two mirror images that are nonidentical is known as enantiomers, which are optically active.
舧 The stereoisomers that are nonsuperimposable on each other and not mirror images of each other are known as diastereomers.
舧 The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds, but they are optically inactive.
舧 Compounds that have a plane of symmetry tend to exist in meso forms. A meso form arises when the two stereoisomers produce superimposable images, and hence, compounds having meso forms are optically inactive.
舧
舧 Aldaric acids (obtained from oxidation of aldohexoses) produce lactones of the order.

舧 Fischer formulated new aldohexose products from glucose by interchanging the end-groups of the
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- A key step in the synthesis of the narcotic analgesic meperidine (trade name Demerol) is the conversion of phenylacetonitrile to X. (a) What is the structure of X? (b) What reactions convert X to meperidine?arrow_forwardAlthough ibuprofen is sold as a racemic mixture, only the S enantiomer acts as an analgesic. In the body, however, some of the R enantiomer is converted to the S isomer by tautomerization to an enol and then protonation to regenerate the carbonyl compound. Write a stepwise mechanism for this isomerization.arrow_forwardMeO 22.55 One potential synthesis of the anti-inflammatory and analgesic drug nabumetone is chloromethylation (Problem 22.48) of 2-methoxynaphthalene followed by an acetoacetic ester synthesis (Section 19.6). 5 3 6 CH₂O CI acetoacetic ester synthesis HC 7 MeO MeO 1 8 2-Methoxynaphthalene Nabumetone (a) Account for the regioselectivity of chloromethylation at carbon 6 rather than at carbon 5 or 7. (b) Show steps in the acetoacetic ester synthesis by which the synthesis of nabum- etone is completed.arrow_forward
- Quinapril (trade name Accupril) is used to treat high blood pressure andcongestive heart failure. One step in the synthesis of quinapril involvesreaction of the racemic alkyl bromide A with a single enantiomer of theamino ester B. Given the structure of quinapril, which one of these two products isneeded to synthesize the drug?arrow_forwardArrange each group of compounds in order of increasing acidity.(b) p-toluenesulfonic acid, acetic acid, chloroacetic acidarrow_forwardEtoposide, sold as a phosphate derivative with the trade name of Etopophos, is used for the treatment of lung cancer, testicular cancer, and lymphomas. (a) Locate the acetals in etoposide. (b) What products are formed when all of the acetals are hydrolyzed with aqueous acid?arrow_forward
- 2. a) Draw the following: (a) nonanamide (b) N-methyloctanamide (c) (d) N-ethyl-N-propylpropanamide N-ethyl-2,4,6-trimethyldecanamidearrow_forward22.47 Tertiary amines with three different alkyl groups are chiral but cannot be resolved because pyramidal inversion causes racemization at room temperature. Nevertheless, chiral aziridines can be resolved and stored at room temperature. Aziridine is a three-membered heterocycle containing a nitrogen atom. The following is an example of a chiral aziridine. In this compound, the nitrogen atom is a chiral center. Suggest a reason why chiral aziridines do not undergo racemization at room temperature.arrow_forwardExplain why an optically active solution of (R)-α-methylbutyrophenone loses its optical activity when dilute acid is added to the solution.arrow_forward
- Practice Problem 19.54 Z Your answer is partially correct. Try again. Predict the major product(s) (A - K) from the treatment of acetone with the following compounds (a-c): NH2 HO Eto OEt A: B: C: D: E: F: OH OH но CN G: H: I: J: (a) [H*], excess EtOH, (-H20) Major Product(s): (ь) NaBH4, Meон B Major Product(s): (c) LAH followed by H20 Major Product(s): SHOW HINTarrow_forwardd-Glucuronic acid is found widely in plants and animals. One of its functions is to detoxify poisonous HO-containing compounds by reacting with them in the liver to form glucuronides. Glucuronides are water soluble and, therefore, readily excreted. After ingestion of a poison such as turpentine or phenol, the glucuronides of these compounds are found in urine. Draw the structure of the glucuronide formed by the reaction of beta-d-glucuronic acid and phenol.arrow_forwardWhen the gum of the shrub Sterculia setigera is subjected to acidic hydrolysis, one of the water-soluble components of thehydrolysate is found to be tagatose. The following information is known about tagatose:(1) Molecular formula C6H12O6(2) Undergoes mutarotation.(3) Does not react with bromine water.(4) Reduces Tollens reagent to give d-galactonic acid and d-talonic acid.(5) Methylation of tagatose (using excess CH3 I and Ag2O) followed by acidic hydrolysis gives1,3,4,5-tetra-O-methyltagatose.(a) Draw a Fischer projection structure for the open-chain form of tagatose.(b) Draw the most stable conformation of the most stable cyclic hemiacetal form of tagatosearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
