
Concept explainers
Propose structures for compounds X, Y, and Z:
X(C7H7Br)NaCN→Y(C8H7N)LiAlH4→Z(C8H11N)
The 1H NMR spectrum of X gives two signals, a multiplet at δ 7.3 (5H) and a singlet at δ 4.25 (2H); the 680-840 cm−1 region of the IR spectrum of X shows peaks at 690 and 770 cm−1. The 1H NMR spectrum of Y is similar to that of X: multiplet at δ 7.3 (5H), singlet at δ 3.7(2H). The 1H NMR spectrum of Z is shown in Fig. 20.7.
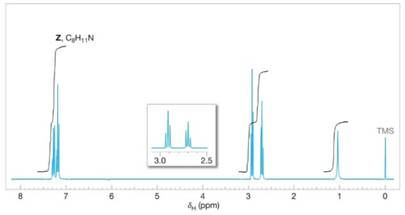
FIGURE 20.7 The 1H NMR spectrum of compound Z. Problem 20.46. Expansion of the signals is shown in the offset plot.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Genetic Analysis: An Integrated Approach (3rd Edition)
Principles of Anatomy and Physiology
Campbell Biology (11th Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry (8th Edition)
Microbiology: An Introduction
- Draw a Newman projection from carbon 3 to carbon 2 in the highest energy conformation for the following molecule. What is this conformation called? What kind of strain is present? Brarrow_forwardWhich of the following dienophiles is most reactive in a Diels-Alder reaction: Please explain why the correct answer to this question is option 5. Please provide a detailed explanation.arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forward
- Draw the enantiomer and diastereomers of the following molecule. Label each type of stereoisomers. Label each chiral center as R or S. HOarrow_forwardWhich diene and dienophile would you choose to synthesize the following compound? Please provide a detailed explanation. Please include a drawing showing the mechanism of the synthesis. Please also explain why it is the correct diene and dienophile.arrow_forwardUsing the sketcher below, draw the structure of N-ethyldecylamine. Answer: 0 ୨୫) . 始 {n [ ]t ?arrow_forward
- Which of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forwardIdentify the characteristic signals that you would expect in the diagnostic region of an IR spectrum of each of the following compounds. a. H₂N b.arrow_forwardWhat is the lowest energy chair for the following cyclohexane? ' || || a. b. " " d.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

