
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20P
Give common or systematic names for each of the following compounds:
(a)
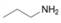
(b)
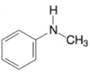
(c)
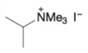
(d)
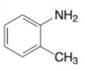
(e)
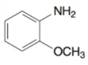
(f)
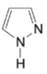
(g)
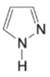
(h)
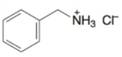
(i)
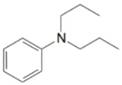
(j)
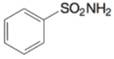
(k)
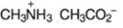
(l)
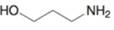
(m)
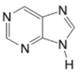
(n)
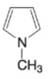
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain how covalent bonds are formed in each of the following compounds in terms of
orbital hybridisation and overlap of orbitals
(i) Ethene, C2H4
(ii) Ethyne, C2H2
Write the contributing resonance structures and resonance hybrid for each of the following:
(a) CH;CH=CH-CH=OH
(f)
CH3
(b) CH2=CH-CH-CH=CH2
(g) CH3-S-CH,
(h) CH3-NO,
(d) CH2=CH-Br
CH,
(e)
What is the correct IUPAC name for HBRO(aq)?
(1) (II) (II)
(IV)
tri-
tetra-
octa- penta-
hepta-
mono-
di-
hexa-
hypobromic oxide
охудen
bromous
hydrogen bromine hydrobromous hypobromous bromic
hydrate acid
Delete
Chapter 20 Solutions
Organic Chemistry
Ch. 20 - Prob. 1PPCh. 20 - Prob. 2PPCh. 20 - Practice Problem 20.3
Write a mechanism that...Ch. 20 - Prob. 4PPCh. 20 - PRACTICE PROBLEM 20.5 Outline a preparation of...Ch. 20 - Prob. 6PPCh. 20 - Prob. 7PPCh. 20 - Prob. 8PPCh. 20 - Prob. 9PPCh. 20 - Prob. 10PP
Ch. 20 - Practice Problem 20.11 In the preceding examples...Ch. 20 - Prob. 12PPCh. 20 - Prob. 13PPCh. 20 - Practice Problem 20.14
Outline a synthesis of...Ch. 20 - Prob. 15PPCh. 20 - Prob. 16PPCh. 20 - Prob. 17PPCh. 20 - Prob. 18PPCh. 20 - Prob. 19PCh. 20 - 20.20 Give common or systematic names for each of...Ch. 20 - Which is the most basic nitrogen in each compound?...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Show how you might synthesize each of the...Ch. 20 - Prob. 25PCh. 20 - 20.26 Provide the major organic product from each...Ch. 20 - Prob. 27PCh. 20 - 20.28 What products would you expect to be formed...Ch. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Write equations for simple chemical rests or state...Ch. 20 - Prob. 33PCh. 20 - Explain the following, including mention of key...Ch. 20 - 20.35 Provide a detailed mechanism for each of the...Ch. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - 20.40 Give structures for compounds R-W:
Ch. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Diethylpropion (shown here) is a compound used in...Ch. 20 - Prob. 44PCh. 20 - 20.45 Compound W is soluble in dilute aqueous HCI...Ch. 20 - 20.46 Propose structures for compounds X, Y, and...Ch. 20 - Compound A(C10H15N) is soluble in dilute HCI. The...Ch. 20 - Prob. 48PCh. 20 - Prob. 49PCh. 20 - For each of the following, identify the product...Ch. 20 - 20.51 Develop a synthesis for the following...Ch. 20 - 20.52 When phenyl isochiocyanatc, , is reduced...Ch. 20 - Prob. 53PCh. 20 - 20.54 Propose a mechanism that can explain the...Ch. 20 - When acetone is treated with anhydrous ammonia in...Ch. 20 - Prob. 56P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Predict the major product of each of the following reactions:
Organic Chemistry As a Second Language: Second Semester Topics
Q5. Which compound is ionic?
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the structures of the compounds 1-3 and explain briefly their formation.arrow_forwardWrite the name of each of the following substances: (Write the answer ONLY) (a) (HCOO)2Ni-H2O (h) CdTe (1) RbzC204 3H2O ) Plarrow_forwardThe degree of unsaturation, or index of hydrogen deficiency, is the number of pi bonds plus rings in a molecule. Specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas: (a) C24H30 (b) C15H22 (c) C,H¡Cl,Narrow_forward
- Which of the following structures are aromatic? CH2 I II II IV (a) I and II (b) III and IV (c) only I (d) І, II and IVarrow_forwardWhich two structures below represent the same compound? (1) (II) (II) (IV) O I and II O Il and III O I and III O I and IVarrow_forwardThe degree of unsaturation, or index of hydrogen deficiency, is the number of pi bonds plus rings in a molecule.Specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas:(a) C9H12(b) C14H18(c) C10H8N2arrow_forward
- The degree of unsaturation, or index of hydrogen deficiency, is the number of pi bonds plus rings in a molecule. Specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas: (a) C22H34 (b) C3H1002 (c) C3H4B13N 6arrow_forwardThe analysis of the structure of compound X which has the molecular formula C14H10O2 provides spectroscopic data as shown below. By systematic analysis, determine the structure of the compound!arrow_forwardThere are two C–C single bonds in penta-1,3-diyne. (a) Which of those bonds would you expect to be stronger? (b) Which of those bonds would you expect to be shorter? (c) The molecule is moderately polar, with a dipole moment of 1.37 D. In which direction would you expect the dipole moment to point? Explain. Penta-1,3-diynearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY