
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18.6, Problem 18.12P
Interpretation Introduction
(a)
Interpretation:
The product formed when the following ammonium salt is treated with
Concept Introduction:
Amines are an organic compound that is derived from ammonia by replacing one or more hydrogen atoms of ammonia by the alkyl groups.- The ammonium ion has hydrogen atoms with a small positive charge. Therefore, the oxygen atom of the hydroxyl ion attacks the hydrogen atom. Then one N-H bond breaks and is bonded to hydroxyl ion to form a water molecule. Thus, the ammonium ion turns into the corresponding amine.
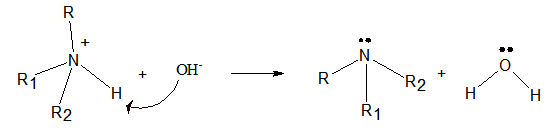
Interpretation Introduction
(b)
Interpretation:
The product formed when the following ammonium salt is treated with
Concept Introduction:
- Amines are organic compound which are derived from ammonia by replacing one or more hydrogen atoms of ammonia by the alkyl groups.
- The ammonium ion has hydrogen atoms with a small positive charge. Therefore, the oxygen atom of the hydroxyl ion attacks the hydrogen atom. Then one N-H bond breaks and is bonded to hydroxyl ion to form a water molecule. Thus, the ammonium ion turns into the corresponding amine.
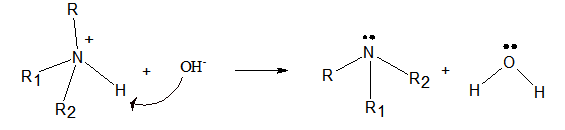
Interpretation Introduction
(c)
Interpretation:
The product formed when the following ammonium salt is treated with
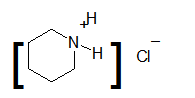
Concept Introduction:
- Amines are organic compound which are derived from ammonia by replacing one or more hydrogen atoms of ammonia by the alkyl groups.
- The ammonium ion has hydrogen atoms with a small positive charge. Therefore, the oxygen atom of the hydroxyl ion attacks the hydrogen atom. Then one N-H bond breaks and is bonded to hydroxyl ion to form a water molecule. Thus, the ammonium ion turns into the corresponding amine.
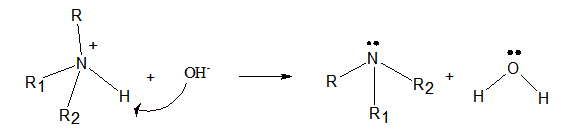
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
CaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254
In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.
The degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chains
Chapter 18 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 18.1 - Prob. 18.1PPCh. 18.1 - Prob. 18.1PCh. 18.1 - Prob. 18.2PCh. 18.1 - Prob. 18.3PCh. 18.2 - Prob. 18.2PPCh. 18.2 - Prob. 18.4PCh. 18.2 - Prob. 18.5PCh. 18.3 - Prob. 18.3PPCh. 18.4 - Decaffeinated coffee is produced by extracting the...Ch. 18.4 - Prob. 18.7P
Ch. 18.5 - Prob. 18.8PCh. 18.5 - Naloxone is a drug used to treat overdoses of...Ch. 18.6 - Prob. 18.4PPCh. 18.6 - Prob. 18.10PCh. 18.6 - Prob. 18.11PCh. 18.6 - Name each ammonium salt. a. ( CH3 NH3)+Cl b. [( CH...Ch. 18.6 - Prob. 18.6PPCh. 18.6 - Prob. 18.12PCh. 18.7 - Prob. 18.13PCh. 18.7 - Prob. 18.14PCh. 18.8 - Prob. 18.15PCh. 18.8 - Prob. 18.16PCh. 18.8 - Prob. 18.17PCh. 18.8 - Prob. 18.18PCh. 18.9 - Prob. 18.19PCh. 18.9 - Prob. 18.20PCh. 18.9 - Prob. 18.21PCh. 18.10 - Prob. 18.22PCh. 18 - Prob. 23PCh. 18 - Prob. 24PCh. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Prob. 29PCh. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - Prob. 32PCh. 18 - Give an acceptable name for each amine. a. b.Ch. 18 - Give an acceptable name for each amine. a. b.Ch. 18 - Give an acceptable name for each amine. a. b. c....Ch. 18 - Give an acceptable name for each amine. a. CH3(...Ch. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - Prob. 39PCh. 18 - Prob. 40PCh. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Prob. 43PCh. 18 - Prob. 44PCh. 18 - Which compound in each pair has the higher boiling...Ch. 18 - Which compound in each pair has the higher boiling...Ch. 18 - Draw the hydrogen-bonding interactions that occur...Ch. 18 - Prob. 48PCh. 18 - Prob. 49PCh. 18 - Which compound has the higher water solubility:...Ch. 18 - Prob. 51PCh. 18 - Prob. 52PCh. 18 - Draw the products of each acid-base reaction. a....Ch. 18 - Draw the products of each acid-base reaction. a....Ch. 18 - Prob. 55PCh. 18 - Prob. 56PCh. 18 - What type of nitrogen heterocycle occurs in both...Ch. 18 - Only one of the N atoms in nicotine has a trigonal...Ch. 18 - Prob. 59PCh. 18 - Prob. 60PCh. 18 - Why are aqueous solutions of an alkaloid slightly...Ch. 18 - Prob. 62PCh. 18 - Prob. 63PCh. 18 - Explain why patients with Parkinson’s disease...Ch. 18 - Prob. 65PCh. 18 - Prob. 66PCh. 18 - Prob. 67PCh. 18 - Prob. 68PCh. 18 - Locate the atoms of 2-phenylethylamine in the...Ch. 18 - Locate the atoms of 2-phenylethylamine in the...Ch. 18 - Give an example of an antihistamine. Explain how...Ch. 18 - Give an example of an anti-ulcer drug, and explain...Ch. 18 - Prob. 73PCh. 18 - Prob. 74PCh. 18 - Prob. 75PCh. 18 - Prob. 76PCh. 18 - Prob. 77PCh. 18 - Prob. 78PCh. 18 - Prob. 79PCh. 18 - Why do some antihistamines cause drowsiness while...Ch. 18 - Prob. 81PCh. 18 - Prob. 82PCh. 18 - Compare the structures of morphine and heroin....Ch. 18 - Prob. 84CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forwardMost ceramic materials have low thermal conductivities because:(a) Electron mobility is strongly restricted due to their strong ionic-covalent bonding.(b) False, in general they are excellent thermal conductors (they are used in ovens).(c) Electron mobility is dependent on T and therefore they are poor conductors at high temperatures.(d) Electron mobility is very restricted by secondary bonds.arrow_forwardResistivity and electrical conductivity.(a) In metals, resistivity decreases.(b) In metals, resistivity decreases and conductivity in semiconductors also decreases with increasing temperature.(c) With increasing temperature, resistivity in metals and conductivity in semiconductors also increases.(d) None of the above.arrow_forward
- State the difference between concrete and Portland cement.(a) There are no differences, in concrete the chemical composition is silicates and in cement aluminates.(b) The chemical composition of concrete is based on silicates and in cement aluminates.(c) Concrete is composed of aggregates bound by cement and cement "only" contains different minerals.(d) Cement is aggregates bound by concrete.arrow_forwardAmorphous polymers are usually transparent and semi-crystalline polymers are usually opaque. Correct?(a) No. They are all made up of polymer chains. True if they were monomers.(b) Yes. The arrangement of the chains determines the passage of light.(c) No. It is the other way around.(d) Crystallinity or amorphousness does not affect the transparency or opacity of the material.arrow_forwardThe name ferrites refers to a family of(a) ceramic materials that exhibit ferrimagnetic behavior due to their ionic composition.(b) polymeric materials that exhibit ferrimagnetic behavior due to their ionic composition.(c) concrete-based materials that exhibit ferrimagnetic behavior due to their ionic composition.(d) superconducting materials that exhibit ferrimagnetic behavior due to their ionic composition.arrow_forward
- State the two main factors affecting ion packing in the solid state.(a) Number of covalent bonds and their unsaturation.(b) Mechanical properties and degradation temperature.(c) Number of crystalline phases present and grain size.(d) Electroneutrality and ion size.arrow_forwardThe ceramic materials alumina (Al2O3) and chromium oxide (Cr2O3) form an isomorphic phase diagram. The solubility will be(a) unlimited of one ceramic in the other.(b) very limited depending on the weight % of Al2O3(c) very limited depending on the weight % of Cr2O3(d) partial of one ceramic in the other.arrow_forwardAmong the main characteristics of optical fibers, indicate which of the following is not included:(a) Opacity and Rigidity(b) Flexibility(c) Transparency(d) Low thicknessarrow_forward
- Most ceramic materials have low thermal conductivities because(a) Electron mobility is strongly restricted due to their strong ionic-covalent bonding.(b) False, in general they are excellent thermal conductors (they are used in ovens).(c) Electron mobility is dependent on T and therefore they are poor conductors at high temperatures.(d) Electron mobility is highly restricted by secondary bonds.arrow_forwardSi increases its conductivity when doped with Ga and P.(a) True, because the conduction mechanism is due to electrons and holes generated by Ga and P as the case may be.(b) True, because a completely different compound is generated.(c) False, because when impurities are introduced, the opposite occurs.(d) False, because the conductivity of Si is only determined by the increase in temperature, which must be controlled.arrow_forwardIndicate whether a configuration and a microstate are the same:a) Yesb) No, a microstate encompasses several configurationsc) No, a configuration is the same as a macrostated) No, a configuration encompasses several microstatesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY