
(a)
Interpretation:
Whether the given molecule is a derivative of 2-phenylethylamine should be stated, if so, the 2-phenylethylamine unit within the molecule should be labeled.
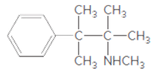
Concept Introduction:
The general structure of 2-phenylethylamine is:
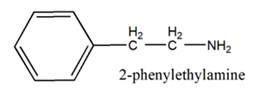
In 2-phenylethylamine, a benzene ring is bonded to a chain of two-carbon atoms that is bonded to a nitrogen atom.
So, the basic unit is of 2-phenylethylamine:
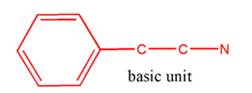
(b)
Interpretation:
Whether the given molecule is a derivative of 2-phenylethylamine should be stated, if so, the 2-phenylethylamine unit within the molecule should be labeled.
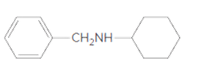
Concept Introduction:
The general structure of 2-phenylethylamine is:
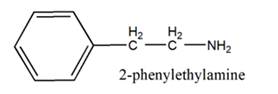
In 2-phenylethylamine, a benzene ring is bonded to a chain of two-carbon atoms that is bonded to a nitrogen atom.
So, the basic unit is of 2-phenylethylamine:
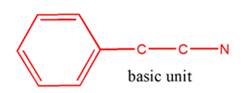
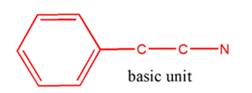
(c)
Interpretation:
Whether the given molecule is a derivative of 2-phenylethylamine should be stated, if so, the 2-phenylethylamine unit within the molecule should be labeled.
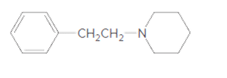
Concept Introduction:
The general structure of 2-phenylethylamine is:
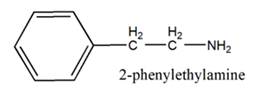
In 2-phenylethylamine, a benzene ring is bonded to a chain of two-carbon atoms that is bonded to a nitrogen atom.
So, the basic unit is of 2-phenylethylamine:
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Which statements are TRUE?I. Tertiary amines have lower BP than primary and secondary amines.II. Tertiary amines has no possibility for hydrogen bonding.III. Tertiary amines has a high-molecular mass as hydrogen bonding occursarrow_forwardWhich of the following statements is true for an amine if "N-" is part of the IUPAC name? a. The compound is a primary amine. b. The molecule is contains a nitrogen atom attached to carbon number one. c. The compound is a secondary amine. d. The compound is a tertiary amine.arrow_forwardWrite structural formulas for these amines. Q.)Cyclohexanaminearrow_forward
- Draw a structural formula for each amine and amine derivative. Q.)1-Phenyl-2-propanamine (amphetamine)arrow_forwardWrite the names for the amines using the naming styles taught in the McMurry text. H2 H3C-N-C-CH3 name: H2 H2 H3C-C-Ņ-c-CH3 H name: These compounds are amines.arrow_forwardamine, (2) an amide, or (3) both an amine and an amide. 17-106 Classify each of the following compounds as (1) an amine, (2) an amide, or (3) both an amine and an NH2 b. `NH a. H2N H d. с.arrow_forward
- 1. Draw the structure for each compound and classify the amine as primary, secondary, or tertiary. a. dimethylamine b. diethylmethylamine c. 2-aminoethanolarrow_forward2. What is produced when an amine reacts with a strong acid such as HCl? A. An amine and the OH- ion B. An amide and the H+ ion C. An ammonium hydroxide D. An ammonium saltarrow_forwardWhich of the following statements is INCORRECT? a. Amines are electrophilic. Amines are stronger bases than alcohols, ethers, or water. Nitrogen atom is sp³-hybridized and is tetrahedral in shape. Solubility of amines decreases with increasing number of carbons. O b. O C. O d.arrow_forward
- What type of amine is N-methylmorpholine? O A. Primary O B. Secondary OC. Tertiary OD. Quaternary O E. None of these choices.arrow_forwarda. 1° amine b. How many nitrogen-hydrogen bonds are present in the functional group in each of the following types of amines? a. 1° amine 17-6 b. 2° amine c. 3° amine Cthe followvingarrow_forwardwhat are the reactions discussed in the Amines/Amides chapterarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
