
(a)
Interpretation:
The ammonium salt which is formed when the given amine is treated with HCl should be determined.
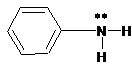
Concept Introduction:
Since
(b)
Interpretation:
The ammonium salt which is formed when the given amine is treated with HCl should be determined.
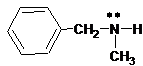
Concept Introduction:
Since amines have a lone pair of electrons on its N atom, they act as proton acceptors. When amines react with an acid, amine accepts protons from acid and forms its conjugate acid. Amines are basic than other organic compounds but are weaker bases than inorganic bases.
(c)
Interpretation:
The ammonium salt which is formed when the given amine is treated with HCl should be determined.
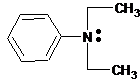
Concept Introduction:
Since amines have a lone pair of electrons on its N atom, they act as proton acceptors. When amines react with an acid, amine accepts protons from acid and forms its conjugate acid. Amines are basic than other organic compounds but are weaker bases than inorganic bases.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
General, Organic, and Biological Chemistry - 4th edition
- The hydrolysis of an amide in acidic conditions forms A. a carboxylate salt and an alcohol B. a carboxylate salt and an amine C. an alcohol and an amine salt (an ammonium ion) D. a carboxylic acid and an amine salt (an ammonium ion)arrow_forwardDraw a structural formula for each amine and amine derivative. Q.) (R)-2-Butanaminearrow_forwardExplain why many amines with useful medicinal properties are sold as their ammonium saltsarrow_forward
- VI. What ammonium salt is formed when each amine is treated with HCI? Draw the structure of the resulting salt. a. -NH2 b. -CH,NHCH3arrow_forwardMatch the description to one of the compounds E– H. a. a compound that contains a 1 ° amine and a 1 ° amide b. a compound that contains a 1 ° amine and a 2 ° amide c. a compound that contains a 2 ° amine and a 3 ° amide d. a compound that contains a 3 ° amine and a 3 ° amidearrow_forwardWhy free bases of alkaloids are not soluble in polor solvent (water) ?arrow_forward
- Give each of the following amines an IUPAC name: a. b. c.arrow_forwardWhat ammonium salt is formed when each amine is treated with HCl? Draw the structure of the resulting salt.arrow_forwardBarbiturates with rapid onset and short duration are: O used to treat epilepsy. O not used medically. O used as anesthetics. less addictive.arrow_forward
- What carbonyl compound and amine are formed by the hydrolysis of each compound?arrow_forwardWhy is the water solubility of a carboxylate salt greater than that of its parent carboxylic acid? A) A carboxylate salt is an ionic compound which is soluble in water. B) A carboxylate salt is less polar than its parent carboxylic acid. C) A carboxylate salt has a lower boiling point than its parent carboxylic acid. D) A carboxylate salt is more readily reduced to an aldehyde than its parent carboxylic acid.arrow_forwardDraw a structural formula for each amine and amine derivative. Q.) Lithium diisopropylamide (LDA)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,


