
Concept explainers
(a)
Interpretation:
Product formed when ethyl benzoate reacts with the given reagent has to be drawn.
Concept Introduction:
Ester Hydrolysis: Ester hydrolysis can be caused by acid and base.
Saponification: Ester hydrolysis taking place in presence of base such as
Acid-catalyzed hydrolysis: In presence of strong acid such as
(a)
Explanation of Solution
The synthesis of product transformation is shown below.
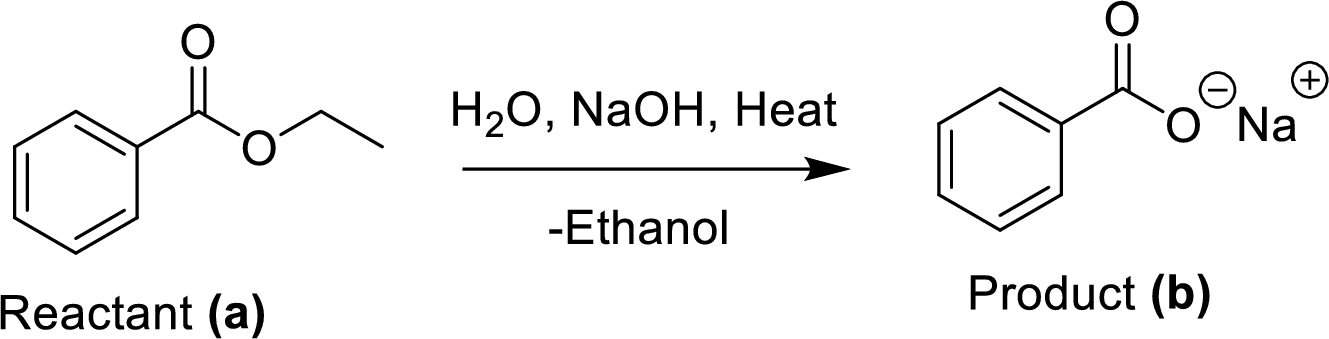
The ethyl benzoate (A) is reacted with sodium hydroxide in presence of basic conditions which corresponding yields the product (B). In this reaction addition and elimination process was occurred.
(b)
Interpretation:
Product formed when ethyl benzoate reacts with the given reagent has to be drawn.
Concept Introduction:
Ester Hydrolysis: Ester hydrolysis can be caused by acid and base.
Saponification: Ester hydrolysis taking place in presence of base such as
Acid-catalyzed hydrolysis: In presence of strong acid such as
(b)
Explanation of Solution
The synthesis of product transformation is shown below.
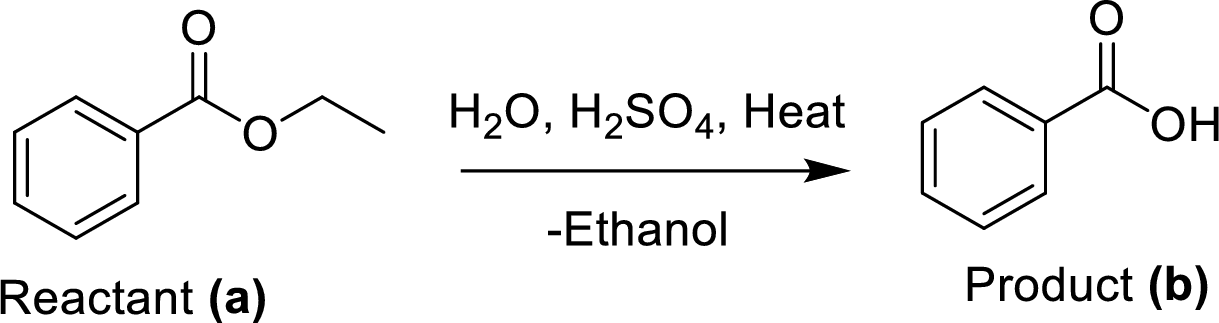
The ethyl benzoate (A) is undergoes for simple acid catalyzed hydrolysis, followed by heating to give a target compound (B), which is a benzoic acid.
(c)
Interpretation:
Product formed when ethyl benzoate reacts with the given reagent has to be drawn.
Concept Introduction:
Amide: One
Amide Formation: Amide is formed when a carboxylic acid reacts with an
- Primary amide is produce when a carboxylic acid reacts with ammonia.
- Secondary and tertiary amide is produce when a carboxylic acid reacts with primary and secondary amine respectively.
(c)
Explanation of Solution
The synthesis of product transformation is shown below.
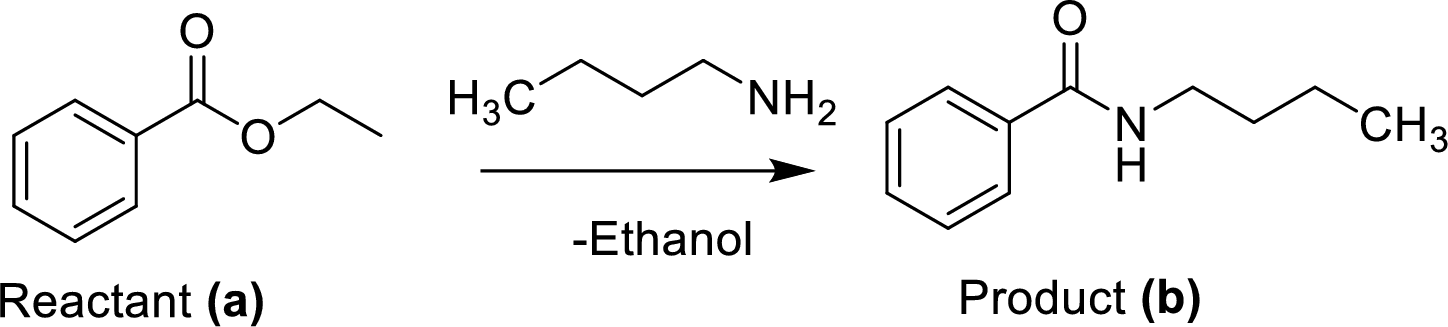
The ethyl benzoate (A) is reacted with n-butylamine in presence of basic conditions which corresponding yields the product (B).
(d)
Interpretation:
Product formed when ethyl benzoate reacts with the given reagent has to be drawn.
Concept Introduction:
Diisobutylaluminium hydride (DIBALH): It is prepared by refluxing triisobutylaluminium in the solvent heptane.
DIBAL-H: is a strong reducing reagent most functional group.
DIBAL-H is a selective reagent (like,
(d)
Explanation of Solution
The synthesis of product transformation is shown below.
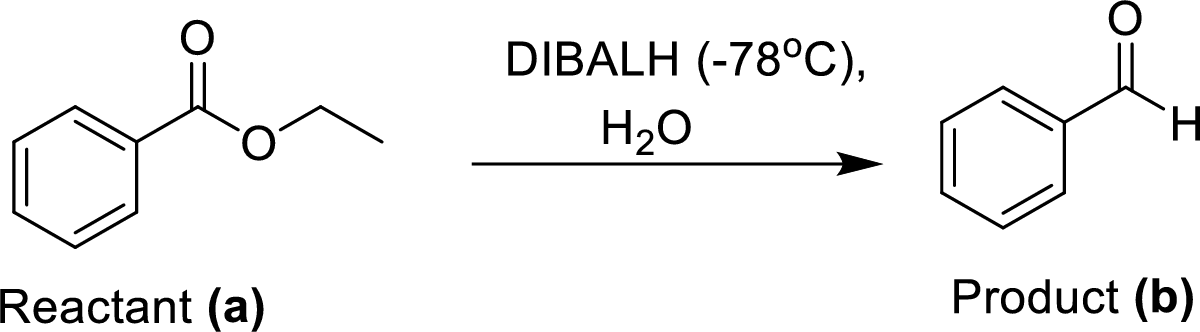
The equal amount of ethyl benzoate (A) is reacted with Diisobutylaluminium hydride (DIBALH) under dry ice conditions at
(e)
Interpretation:
Product formed when ethyl benzoate reacts with the given reagent has to be drawn.
Concept Introduction:
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
LAH Reduction: The saturated/unsaturated aldehyde and ketones in the presence of sodium metal in LAH and carbonyl compound produced saturated alcohols. The keto group involves in the reduction process of LAH, this end up reducing to give the alcohols.
(e)
Explanation of Solution
The synthesis of product transformation is shown below.
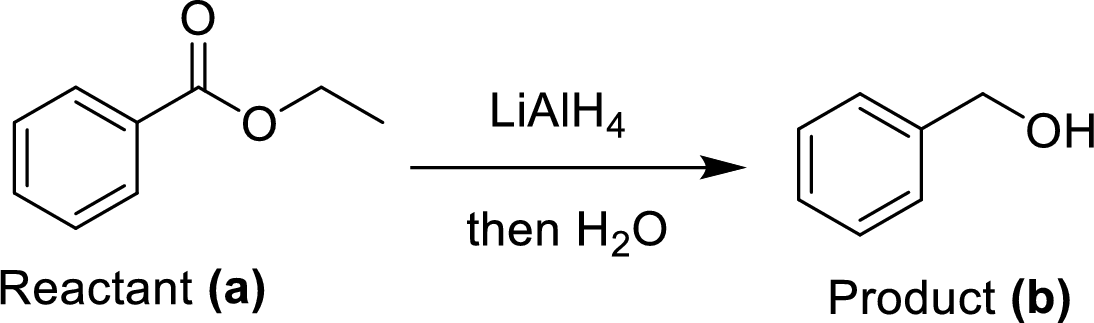
The ethyl benzoate (A) undergoes for LAH reduction process followed by simple hydrolysis workup method to give a target product (B). The obtained product namely benzyl alcohol.
(f)
Interpretation:
Product formed when ethyl benzoate reacts with the given reagent has to be drawn.
Concept Introduction:
Alkyl or aryl magnesium halides (
Synthesis of Grignard reagent is shown below,
Acid Catalyzed Hydration Reaction: The reaction involves breaking of
(f)
Explanation of Solution
The synthesis of product transformation is shown below.
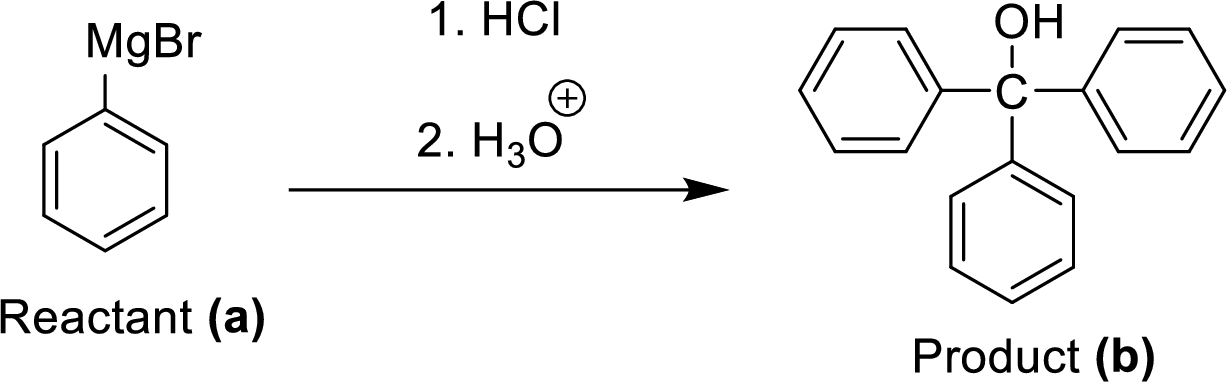
Two equivalents of Grignard reagent (A) is reacted with hydrogen chloride and fallowed by hydrolysis workup method, which corresponding yields the triphenyl methanol (B) it is a target molecule.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) a) Given 7.70 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? b) A chemist ran the reaction and obtained 5.25 g of ethyl butyrate. What was the percent yield? c) The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.70 g of butanoic acid and excess ethanol?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.50 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%yield? Express your answer in grams to three significant figures.arrow_forward
- How could you convert butanenitrile into the following compounds? Write each step showing the reagents needed. (a) 1-Butanol (b) Butylaminearrow_forward(a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardComparing Hydration Products Using Two Different Methods Draw the product formed when CH3CH2C=CH is treated with each of the following sets of reagents: (a) H2O, H2SO4, HgSO4; and (b) R2BH, followed by H2O2, HO−.arrow_forward
- (a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardGive a systematic (IUPAC) name for each diol.(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3 (b) HO¬(CH2)8¬OHarrow_forwardPredict the products formed when cyclohexanone reacts with the following reagents.(a) CH3NH2, Harrow_forward
- 1. Draw structures corresponding to the following IUPAC names: (a) 4-Methylpentanoic acid (b) o-Hydroxybenzoic acid (c) 2,2-Dimethylpropanoyl chloride (d) trans-2-Methylcyclohexanecarboxamide (e) p-Methylbenzoic anhydride (f) p-Bromobenzonitrilearrow_forwardDraw a structural formula for the product formed by treating butanal with each reagent. (a) LiA1H4LiA1H4 followed by H2OH2O (b) NaBH4NaBH4 in CH3OH/H2O (c) H2/Pt (d) Ag(NH3)2+in NH3/H2O (e) H2CrO4, heat (f) HOCH2CH2OH,HClarrow_forwardPhenylethanol can be oxidised to phenylethanal or phenylethanoic acid, depending on the reagents used (both the alcohol and the aldehyde are of interest for their antimicrobial properties, while the acid is used to treat type II hyperammonemia): A (a) (b) (c) CoH,CH,CHO phenylethanal B C6H5CH₂CH₂OHC₂H₂CH₂CO₂H phenylethanol Suggest reagents (shown as A and B in the scheme above) that could be used to carry out the oxidation of the alcohol to the aldehyde and the acid, respectively. C6H5CH₂- Suggest two other syntheses of phenylethanoic acid, in each case indicating the starting materials and other reagents required, but not giving details of mechanism. One of your proposed syntheses must start with a compound which only contains seven carbon atoms (the acid product contains eight carbon atoms). phenylethanoic acid Phenylethanal can be converted to a hydrate in the presence of aqueous acid, though the position of equilibrium is very far to the left: H H+/H₂O OH C6H5CH₂-C-H OH Explain why…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





