
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16.2, Problem 16.1P
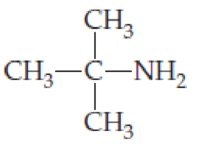
Identify the following compounds as primary, secondary, or tertiary
- (a) CH3(CH2)4CH2NH2
- (b) CH3CH2CH2NHCH(CH3)2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the skeletal structure of the alkene that is needed as a starting material to prepare each of the following alcohols.
Part 1 of 2
CH3CH(OH)CH3
Click and drag to start drawing a
structure.
☑
:
What is the IUPAC name of the following compound? (No need to provide E/Z
designation.)
CO₂H
The reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?
Chapter 16 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 16.2 - Identify the following compounds as primary,...Ch. 16.2 - Prob. 16.2PCh. 16.2 - Prob. 16.3PCh. 16.2 - Prob. 16.4PCh. 16.2 - Prob. 16.5KCPCh. 16.2 - Prob. 16.6KCPCh. 16.3 - Arrange the following compounds in order of...Ch. 16.3 - Draw the structures of (a) ethylamine and (b)...Ch. 16.4 - Provide compounds that fit the following...Ch. 16.4 - Prob. 16.10P
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Write an equation for the acid-base equilibrium...Ch. 16.5 - Prob. 16.13PCh. 16.5 - Prob. 16.14PCh. 16.5 - Prob. 16.15PCh. 16.5 - Prob. 16.16PCh. 16.6 - Prob. 16.17PCh. 16.6 - Prob. 16.18PCh. 16.6 - Prob. 16.19PCh. 16.6 - Prob. 16.20PCh. 16.6 - Prob. 16.21PCh. 16.6 - Prob. 16.22PCh. 16.7 - Prob. 16.1CIAPCh. 16.7 - Prob. 16.2CIAPCh. 16.7 - Prob. 16.3CIAPCh. 16 - (a) For the compound above, identify each nitrogen...Ch. 16 - The structure of the amino acid lysine (in its...Ch. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - Prob. 16.27UKCCh. 16 - Complete the following equations: (a) (b)...Ch. 16 - Prob. 16.29APCh. 16 - Draw the structures corresponding to the following...Ch. 16 - Name the following amines, and classify them as...Ch. 16 - Name the following amines, and identify them as...Ch. 16 - Prob. 16.33APCh. 16 - Which is a stronger base, diethyl ether or...Ch. 16 - Prob. 16.35APCh. 16 - Prob. 16.36APCh. 16 - The compound lidocaine is used medically as a...Ch. 16 - Prob. 16.38APCh. 16 - Draw the structures of the ammonium ions formed...Ch. 16 - Prob. 16.40APCh. 16 - Prob. 16.41APCh. 16 - Prob. 16.42APCh. 16 - Prob. 16.43APCh. 16 - Prob. 16.44APCh. 16 - Prob. 16.45CPCh. 16 - Prob. 16.46CPCh. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48CPCh. 16 - How do amines differ from analogous alcohols in...Ch. 16 - Name at least two undesirable characteristics are...Ch. 16 - Prob. 16.52CPCh. 16 - Complete the following equations (Hint: Answers...Ch. 16 - Prob. 16.54CPCh. 16 - Prob. 16.55CPCh. 16 - Why is cyclohexylamine not considered to be a...Ch. 16 - Prob. 16.57CPCh. 16 - Prob. 16.58GPCh. 16 - 1-Propylamine, 1-propanol, acetic acid, and butane...Ch. 16 - Prob. 16.60GPCh. 16 - Lemon juice, which contains citric acid, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- When pentane is exposed to Br2 in the presence of light, a halogenation reaction occurs. Write the formulas of:(a) All possible products containing only one bromine(b) All possible products containing two bromines that are not on the same carbonarrow_forwardWrite the IUPAC name for each unsaturated hydrocarbon. (a) CH,=CH(CH,),CH3 CH3 CH3 H,C- (b) CH3 (c) CH3 alaTTarrow_forwardDraw the skeletal structure of the alkene that is needed as a starting material to prepare each of the following alcohols. Part 1 of 2 CH3CH2CH2CH2OH Click and drag to start drawing a structure. ☑ :☐arrow_forward
- 2-butanol can be formed as the only product of the Markovnikov addition of H2O to two different alkenes. In contrast, 2-pentanol can be formed as the only product of the Markovnikov addition of H2O to just one alkene. To examine the difference, draw the alkene starting materials of each alcohol. : Draw the bond-line (skeletal) structures of the two alkene starting materials that can be used to synthesize 2-butanol via Markovnikov hydration. Part 1 of 2 Click and drag to start drawing a structure. ☑arrow_forwardDrawn are four isomeric dimethylcyclopropanes. How are the compounds in each pair related (enantiomers, diastereomers, constitutional isomers): A and B; A and C; B and C; C and D?arrow_forwardWhich compounds are enantiomers?arrow_forward
- b) Determine which ionization state corresponds to aspartic acid (shown below) at pH 1, 7, and 10 by selecting the most suitable answer from the statements i-iv. The pK of the amine, carboxylic acid, and side chain of aspartic acid are 9.6, 1.88 and 3.65, respectively. ОН ÓH ÑH2 i) the amine is charged; the carboxylic acids are neutral. ii) the amine and carboxylic acids are all charged. iii) the amine is neutral, and the carboxylic acids are charged. iv) the amine and the carboxylic acids are all neutral. pH Statement (i, ii, iii, or iv) 1 7 10 %3Darrow_forwardThe structure for 2-chloro-3-heptene is:arrow_forwardDraw the structures of the following compounds. (Includes both new and old names.) 3-cyclopentylhexan-3-olarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license