
Concept explainers
(a)
Interpretation:
The organic reactant in the given reaction equation has to be named.
Concept introduction:
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
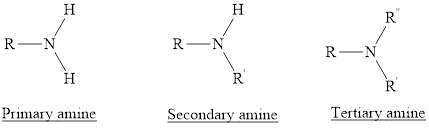
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Amines form salts by accepting a proton from strong mineral acids.
(b)
Interpretation:
The organic reactant in the given reaction equation has to be named
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Reaction of amines and acid will give amine salt an (ammonium ion).
Treating an amine salt with a strong base regenerates the “parent” amine.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(c)
Interpretation:
The organic reactant in the given reaction equation has to be named
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Amines form salts by accepting a proton from strong mineral acids.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- 49.10 Lactic Acid Salicylic Acid 48 5 g 2g Trichloroacetic Acid Flexible Collodion q.s. ad 100 g Sig: wart remover. Use as directed. (a) Flexible collodion contains 20% w/w camphor and 30% w/w castor oil. How many grams of each would be contained in 30 g of the mixture? (b) The specific gravity of castor oil is 0.955. How many milliliters of the oil are contained in 30 g of the mixture? (c) If the specific gravity of the mix- ture is 0.781, what are the w/v con- centrations of lactic acid, salicylic acid, and trichloroacetic acid in the mixture? 49. (a) 5.34 g camphor and 8.01 g castor oil (b) 8.39 mL castor oil (c) 3.12% w/v lactic acid, 3.91% w/v sali- cylic acid, and 1.56% w/v trichloroacetic acidarrow_forwardName the following compound and indicate whether or not is a reducing sugar:arrow_forwardWhat structural feature is necessary for an alcohol to undergo oxidation reactions?arrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
