
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16, Problem 16.28UKC
Complete the following equations:
(a) 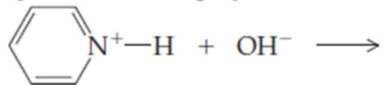
(b) 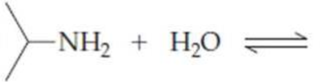
(c)
(d) 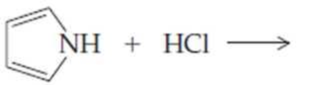
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Two solutions, 250.0 mL of 1.00 M CaCl2(aq) and 250.0 mL of 1.00 M K2SO4(aq), are combined, and the temperature decreased by 2.40 degrees C. Determine qrxn per mole of CaSO4(s) formed in the reaction.
A) +12.0 kJ/mol
B) -12.0 kJ/mol
C) +6.00 kJ/mol
D) -6.00 kJ/mol
Write a balanced equation for each of the following singlereplacement reactions. (a) Zinc granules are added to carbonic acid. (b) Cadmium metal is added to acetic acid.
Consider the reaction A + 2B ----> C. If the molar mass of C is twice the molar mass of A, what mass of C is produced by the complete reaction of 10.0 g A?(a) 10.0 g(b) 30.0 g(c) 60.0 g
Chapter 16 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 16.2 - Identify the following compounds as primary,...Ch. 16.2 - Prob. 16.2PCh. 16.2 - Prob. 16.3PCh. 16.2 - Prob. 16.4PCh. 16.2 - Prob. 16.5KCPCh. 16.2 - Prob. 16.6KCPCh. 16.3 - Arrange the following compounds in order of...Ch. 16.3 - Draw the structures of (a) ethylamine and (b)...Ch. 16.4 - Provide compounds that fit the following...Ch. 16.4 - Prob. 16.10P
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Write an equation for the acid-base equilibrium...Ch. 16.5 - Prob. 16.13PCh. 16.5 - Prob. 16.14PCh. 16.5 - Prob. 16.15PCh. 16.5 - Prob. 16.16PCh. 16.6 - Prob. 16.17PCh. 16.6 - Prob. 16.18PCh. 16.6 - Prob. 16.19PCh. 16.6 - Prob. 16.20PCh. 16.6 - Prob. 16.21PCh. 16.6 - Prob. 16.22PCh. 16.7 - Prob. 16.1CIAPCh. 16.7 - Prob. 16.2CIAPCh. 16.7 - Prob. 16.3CIAPCh. 16 - (a) For the compound above, identify each nitrogen...Ch. 16 - The structure of the amino acid lysine (in its...Ch. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - Prob. 16.27UKCCh. 16 - Complete the following equations: (a) (b)...Ch. 16 - Prob. 16.29APCh. 16 - Draw the structures corresponding to the following...Ch. 16 - Name the following amines, and classify them as...Ch. 16 - Name the following amines, and identify them as...Ch. 16 - Prob. 16.33APCh. 16 - Which is a stronger base, diethyl ether or...Ch. 16 - Prob. 16.35APCh. 16 - Prob. 16.36APCh. 16 - The compound lidocaine is used medically as a...Ch. 16 - Prob. 16.38APCh. 16 - Draw the structures of the ammonium ions formed...Ch. 16 - Prob. 16.40APCh. 16 - Prob. 16.41APCh. 16 - Prob. 16.42APCh. 16 - Prob. 16.43APCh. 16 - Prob. 16.44APCh. 16 - Prob. 16.45CPCh. 16 - Prob. 16.46CPCh. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48CPCh. 16 - How do amines differ from analogous alcohols in...Ch. 16 - Name at least two undesirable characteristics are...Ch. 16 - Prob. 16.52CPCh. 16 - Complete the following equations (Hint: Answers...Ch. 16 - Prob. 16.54CPCh. 16 - Prob. 16.55CPCh. 16 - Why is cyclohexylamine not considered to be a...Ch. 16 - Prob. 16.57CPCh. 16 - Prob. 16.58GPCh. 16 - 1-Propylamine, 1-propanol, acetic acid, and butane...Ch. 16 - Prob. 16.60GPCh. 16 - Lemon juice, which contains citric acid, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- A student ran the following reaction in the laboratory at 759 K: N2(g) + 3H2(g) = 2NH3(g) When she introduced 3.13×10-2 moles of N2(g) and 6.00x102 moles of H2(g) into a 1.00 liter container, she found the equilibrium concentration of NH3(g) to be 6.84×10-4 M. Calculate the equilibrium constant, K., she obtained for this reaction. K. =arrow_forwardBalance the following (fictional) chemical equations by supplying the correct coefficient. Do not leave any space blank (in other words, write in 1 if you would be leaving it blank): R(OZ)2 - RY2+ Z20 a) ZY+ b) D2 (g) + L2 (g) – DL3 (e)arrow_forwardConsider these compounds: A. PbBr, B. MnS C. Ag,CO3 D. AIPO, Complete the following statements by entering the letter(s) corresponding to the correct compound(s). (If more than one compound fits the description, include all the relevant compounds by writing your answer as a string of characters without punctuation, e.g, ABC.) Without doing any calculations it is possible to determine that magnesium fluoride is more soluble than and magnesium fluoride is less soluble than| It is not possible to determine whether magnesium fluoride is more or less soluble than by simply comparing Kgp values.arrow_forward
- Consider the following reaction at 25°C with the ΔG°’ = +1800 J/mol for the forward reaction.The molar concentrations at the beginning of the reaction were [A] = 19 mM and [B] = 10 mM.After 1 hour, the concentrations were [A] = 16 mM and [B] = 13 mM. Calculate the ΔG of the reaction at the 1 hour timepoint. Please round to 1 decimal point.Gas constant = 8.315 J/mol Karrow_forwardGiven the following electrode reactions: 2+ Co +e + Co E=1,92 V 3+ Co(NH,), + e + Co(NH,), E=0,06 a. Is it possible to use an iron wire for the determination of ammonia? How would you do it if it were possible? b. What order would the electrode be, relative to ammonia? c. If the answer is yes, derive the Nernst equation for the electrode. If the answer is negative, explain why.arrow_forwardFill in the blanks: Identify the oxidizing and reducing agent of the given equation: Answers should be the symbol of the element only. 8H*(aq) + 6Cl(aq) + Sn(s) + 4NO3-(aq) SnCl²(aq) + 4NO₂(g) + 4H₂O(1) 1. oxidizing agent 2. reducing agent = Sarrow_forward
- For each of the following reactions, give a balanced net-ionic equation. Sulfate Ion a) MgSO4 + H2SO4 b) MgSO4 + BaCl2 Sulfite Ion a) Na2SO3 + H2SO4 b) Na2SO3 + BaCl2 c) BaSO3 + HNO3 Iodide Ion a) NaI + NaOCl b) NaI + AgNO3 c) AgI + NH3 d) NaI + H2SO4arrow_forwardThe main constituents in vinegar are water and ethanoic acid (CH3COOH). In order to determine the concentration of acid in homemade vinegar, a student titrated 25 cm3 of 001 M NaOH against the vinegar. The equation for the reaction is: CH3COOH(aq) + NaOH(aq) ® CH3COONa(aq) + H2O(l) The following titration results were obtained: Burette readings (cm3) Rough 1 2 Final burette reading 20.10 38.90 31.40 Initial burette reading 0.10 20.00 12.50 Volume of vinegar used 20.00 18.90 18.90 (a) What volume of vinegar should be used in the calculation? (b) What is the mole ratio of NaOH:CH3COOH? (c) Calculate the number of moles of alkali in 25 cm3 of NaOH solution used. (d) How many moles of acid were used in the titration? (e) Calculate the…arrow_forwardAcetic acid is the principal ingredient in vinegar as shown; that's why it tastes sour. At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and [H3 O+] = [CH3 CO2−] = 0.00118 M. What is the value of Ka for acetic acid?arrow_forward
- Draw a structure for the compound, C3H5Br, that fits the following 1H NMR data: δ 2.32 (3H, singlet) δ 5.35 (1H, broad singlet) δ 5.54 (1H, broad singlet)arrow_forwardCao (s) (calcium oxide) is the main ingredient of concrete. While mixing Cao with water, the mixture gets hotter rapidly by its own. The chemical equation of the reaction is given below. Cao (s) + H20 (liq) > Ca(OH)2 (s) Based on the above information, which of the following is correct for the above process to mix Cao (s) with water. O a. AH> 0, AS> 0, AG0, AG 0 , ΔS> 0 , ΔG>0 O e. AH0, AG>0 O f. AH 0arrow_forwardPotassium superoxide, KO2, is used in rebreathing masks to generate oxygen according to the reaction below. If the mask contains 0.250 mol KO2 and 0.200 mol water, what is the limiting reagent? How many moles of excess reactant will there be once the reaction is complete? 4 KO2(s) + 2 H2O(ℓ) → 4 KOH(s) + 3 O2(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license