
Concept explainers
(a)
Interpretation: Considering the base hydrolysis of given ester answer the follwoing questions.
(a) The product which contains labelled oxygen atom
Concept introduction:
The hydrolysis reaction of an ester can be catalyzed in presence of a base that is hydroxide ion. The
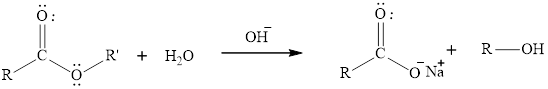
Here, the ester molecule contains a labelled oxygen atom
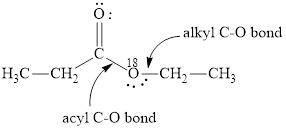
(b)
Interpretation:
Considering the base hydrolysis of given ester answer the follwoing questions.
(b) The product which contains labelled oxygen atom
Concept introduction:
The hydrolysis reaction of an ester can be catalyzed in presence of a base that is hydroxide ion. The rate of reaction increase due to the presence of hydroxide ion which can act as better nucleophile than water molecule. Thus substitution of alkoxide group takes place and carboxylic acid formation occur. The general reaction of base hydrolysis of en ester is written as,
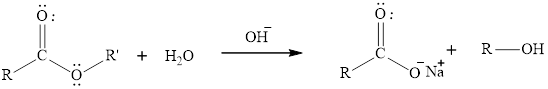
Here, the ester molecule contains a labelled oxygen atom

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
- Which reagents are best used to produce 4-methyl-2-pentanol from a ketone? a. amins and trace acid, followed separetely H2/PD/C b. sodium cyanide followed by HCl c. sodium borohydridd followed by acidic work up d. hydrogen cyanide followed by HCl e. none of the abovearrow_forwardFill in the appropriate reagents for the following reaction:arrow_forward4. Draw a chemical reaction scheme that includes an arrow - pushing mechanism for each of the following reactions: a. Protonation of p-acetyl aniline by HCI followed by deprotonation with NaOH b. Deprotonation of benzoic acid by NaOH followed by protonation with HCIarrow_forward
- Below is a structure of Levonorgestrel (Plan B®), used in prevention of pregancy. но HH H. Levonorgestrel Plan B® Below is one of the reactions used in the synthesis. What type of reaction is it? он :CEC-H Lc=c-H THF as solvent Levonorgestrel H,CO H,CO O A grignard reaction. Nucleophilic acyl substitution. O Nucleophilic addition. O An SN2 reaction. O Oarrow_forward7. Only one of the following carboxylic acids can be made via a malonic ester synthesis. Which one is it? Ph A Ph. OH OH Ph OH OH B C D OH Earrow_forwardWhich alkyl halides were used in the acetoacetic ester synthesis to make the substituted ketone below? You may choose more than one answer. CH3BR CH3CH2CH2Br СНЗСН2Br O (CH3)2CHB.arrow_forward
- 12 Why is ethanal a stronger acid than ethane? CH3-C-H ethanal CH3-CH3 ethane A. The conjugate base of ethanal (enolate ion) is stabilized by resonance; thus, the compulsion of ethanal to lose a H* (forming the enolate) is increased. Ethane's conjugate base is not stabilized. B. The inductive electron withdrawing effect of the C=O in ethanal increases its proclivity to release a H* C. Steric factors make ethanal the stronger acid. D. You can write resonance structures for ethanal (the carbon acid), indicating enhanced stability. Because the carbon acid is more stable, its acid strength is greater.arrow_forwardQuestion 2 a) i) Draw the structure of the product obtained when the following diester X undergoes an intramolecular Dieckmann condensation. Diester X ii) Write a complete mechanism for the reaction.arrow_forwarda) Ketoacid Z below can be synthesized from ethyl acetoacetate W via an acetoacetic ester synthesis. The B-ketoester W can be initially synthesized by Claisen condensation reaction of ester V. NaOEt NaOEt Base Ester V OEt CH,Br OEt | HO CH, Ketoacid Z i) Identify the structure of ester V. ii) Transformation of W to X and X to Y are alkylation reactions. Draw the structures of the intermediates X and Y. Determine a suitable base for the conversion of X to Y. iii) With regards to the formation of the ketoacid Z from Y, show the steps involved in the reaction by giving the appropriate intermediates, reagents and reaction conditions. iv) Explain why only one of the carbonyl groups in Y is lost as carbon dioxide gas.arrow_forward
- The reaction of a nitrile with an alcohol in the presence of a strong acid forms an N-substituted amide. This reaction, known as the Ritter reaction, doesnot work with primary alcohols. a. Propose a mechanism for the Ritter reaction.b. Why does the Ritter reaction not work with primary alcohols?c. How does the Ritter reaction differ from the acid-catalyzed hydrolysis of a nitrile to form an amide?arrow_forwardChoose the best reagents to complete the following reaction. $ H₂O OH $ < OH A B C D E NaOH H₂O H2, Raney nickel TSOH, H₂O (CH2OH)2, TSOH Donearrow_forwardKetones are converted to enolates on treatment with base. Draw the structures of the enolates formed when the ketone shown is treated with either LDA or tert-butaxide. Include all lone pairs of electrons and nonzero formal charges. Do not include counter ions. 010 H,C i See Periodic Table o Se Hint Part 1 Drawthe enolate formed when the ketone is treated with LDA: Select a tool to begin drawing Mann 5 Part 2 Draw the enolate formed when the ketone is treated with tert-butoxide: + I : 0arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

