
(a)
Interpretation:
To explain the following result obtain from theHammett equation.
Concept introduction:
Hammett reaction constant (
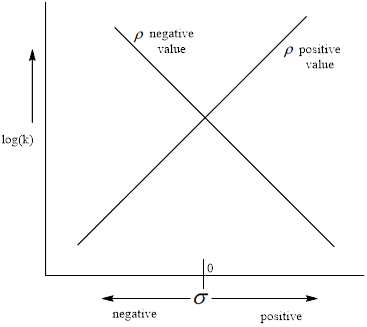
The negative value of the
(b)
Interpretation:
To explain the following result obtain from theHammett equation.
Concept introduction:
Hammett reaction constant (

The negative value of the
(c)
Interpretation:
To explain the following result obtain from theHammett equation.
Concept introduction: Hammett reaction constant (

The negative value of the
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- Electrophilic aromatic substitution usually occurs at the 1-position of naphthalene, also called the a position. Predict the major products of the reactions of naphthalene with HNO3, H2SO4.arrow_forwardWhen ethoxybenzene is treated with a mixture of nitric acid and sulfuric acid, two products are obtained, each of which has the molecular formula C8H9NO3. For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly in your products. Do not use abbreviations such as Me or Ph.arrow_forwardPeroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homolytically rather easily. For example, the bond-dissociation enthalpy of the O¬O bond in hydrogen peroxide (H¬O¬O¬H) is only 213 kJ>mol (51 kcal>mol). Give a mechanism for the hydrogen peroxide-initiated reaction of cyclopentane with chlorine. The BDE for HO¬Cl is 210 kJ>mol (50 kcal>mol).arrow_forward
- When the three benzaldehydes shown below were exposed to HCN under identical conditions it was found that the equilibrium constants, K, for the three cyanohydrin formation reactions were different. Based on principles that govern equilibrium processes for reversible carbonyl addition reactions, place the K values for the three reactions in ascending order (smallest to largest). Briefly justify your answer. (Hint: Recall that electron deficient carbonyls tend to react faster with nucleophiles and have larger equilibrium constants for the addition reaction.) H X O + HCN pH ~ 9-10 X = H, NO₂, OCH 3 OH H+CN Xarrow_forwardThe pKa values of a few ortho-, meta-, and para-substituted benzoic acids are shown below: The relative pKa values depend on the substituent. For chloro-substituted benzoic acids, the ortho isomer is the most acidic and the para isomer is the least acidic; for nitro-substituted benzoic acids, the ortho isomer is the most acidic and the meta isomer is the least acidic; and for amino-substituted benzoic acids, the meta isomer is the most acidic and the ortho isomer is the least acidic. Explain these relative acidities.a. Cl: ortho 7 meta 7 para b. NO2: ortho 7 para 7 meta c. NH2: meta 7 para 7 orthoarrow_forwardThe pKa values of a few ortho-, meta-, and para-substituted benzoic acids are shown below: The relative pKa values depend on the substituent. For chloro-substituted benzoic acids, the ortho isomer is the most acidic and the para isomer is the least acidic; for nitro-substituted benzoic acids, the ortho isomer is the most acidic and the meta isomer is the least acidic; and for amino-substituted benzoic acids, the meta isomer is the most acidic and the ortho isomer is the least acidic. Explain these relative acidities. a. Cl: ortho > meta > para b. NO2: ortho > para > meta c. NH2: meta > para > orthoarrow_forward
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardWhen 2-bromo-2-methylbutane is treated with a base, a mixture of 2-methyl-2-butene and 2-methyl-1- butene is produced. When potassium hydroxide is the base, 2-methyl-1-butene accounts for 45% of the product mixture. However, when potassium tert-butoxide is the base, 2-methyl-1-butene accounts for 70% of the product mixture. What percent of 2-methyl-1-butene would be in the mixture if potassium propoxide were the base? base Br A. Less than 45% B. C. 45% Between 45% and 70% D. More than 70%arrow_forwardEthers can be prepared by reaction of an alkoxide or phenoxide ion with a primary alkyl halide. Draw the structure of the expected organic product of the reaction of iodoethane with the following alkoxide ion: H3C CH3 + Na You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. Do not include lone pairs in your answer. They will not be considered in the grading. • Do not include counter-ions, e.g., Na", I, in your answer. P opy aste [*arrow_forward
- Furan undergoes electrophilic aromatic substitution more readily than benzene; mild reagents and conditions are sufficient.For example, furan reacts with bromine to give 2-bromofuran. Explain why furan undergoes bromination (and other electrophilic aromatic substitutions) primarily at the 2-position.arrow_forwardThe following substances can be prepared by a nucleophilic addition reaction between an aldehyde or ketoneand a nucleophile. Identify the reactants from which they were prepared. If the substance is an acetal, identifythe carbonyl compound and the alcohol; if it is an imine or enamine, identify the carbonyl compound and theamine. You do not have to consider stereochemistry. In cases where there is more than one answer, just giveone. Use Grignard reagents when an organometallic reagent is required. Draw the Grignard reagent as acovalent magnesium bromide.arrow_forwardThe nitroso group, −N=O, is one of the few nonhalogens that is an ortho- and para-directing deactivator. Explain this behaviour by drawing resonance structures of the carbocation intermediates in ortho, meta, and para electrophilic reaction onnitrosobenzene, C6H5−N=O.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

