
Concept explainers
(a)
Interpretation:
IUPAC name for the fumaric acid has to be given.
Concept Introduction:
For naming a
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for
aldehyde , the carboxyl carbon is always numbered 1. - The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl
functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix. - If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(a)
Answer to Problem 16.34EP
IUPAC name of fumaric acid is trans-butenedioic acid.
Explanation of Solution
Structure of fumaric acid is,
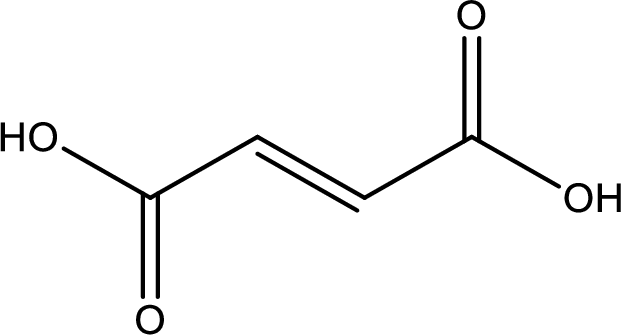
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The structure contains a double bond in it. The parent carbon chain is butene. The given structure contains two carboxyl groups. The carboxylic acid is named by adding suffix “-dioic acid”. This gives the name of carboxylic acid as butenedioic acid.
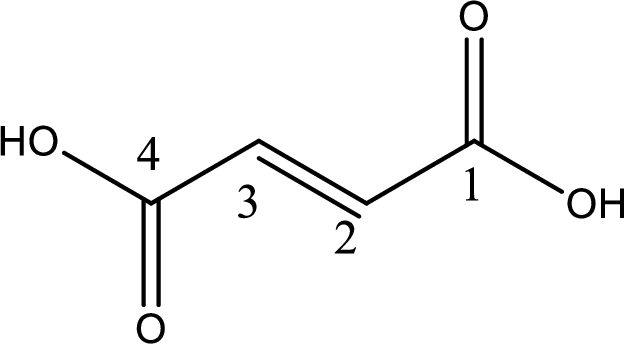
Looking for substituents it is found that there are no substituents present in the carbon chain. Stereochemistry is possible across the double bond. As the two hydrogen atoms are on the opposite side of double bond, the configuration at the double bond is “trans”. This has to be included in the name to get the IUPAC name. IUPAC name of the fumaric acid is found as trans-butenedioic acid.
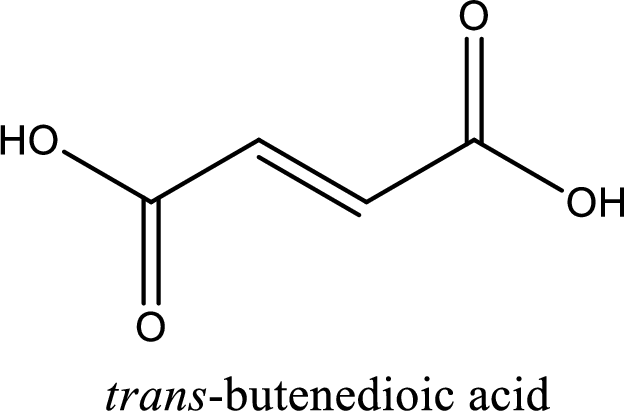
IUPAC name of fumaric acid is given.
(a)
Interpretation:
IUPAC name for the pyruvic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(a)
Answer to Problem 16.34EP
IUPAC name of pyruvic acid is 2-oxopropanoic acid.
Explanation of Solution
Structure of pyruvic acid is,
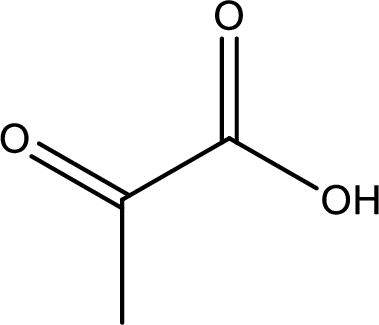
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a three carbon chain. The parent alkane is propane. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as propanoic acid.
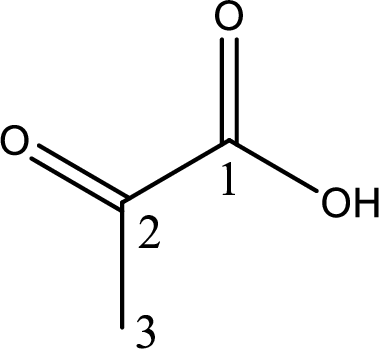
Looking for substituents it is found that there is a keto group present on the second carbon atom. Hence, the IUPAC name of the pyruvic acid is 2-oxopropanoic acid.
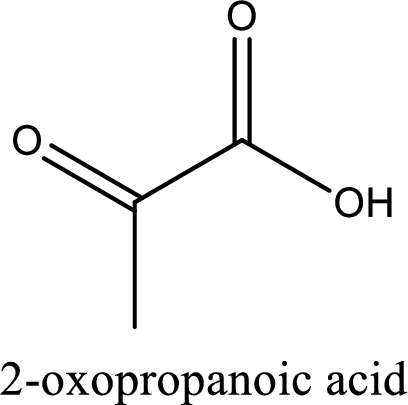
IUPAC name of pyruvic acid is given.
(c)
Interpretation:
IUPAC name for the malic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(c)
Answer to Problem 16.34EP
IUPAC name of malic acid is 2-hydroxybutanedioic acid.
Explanation of Solution
Structure of malic acid is,
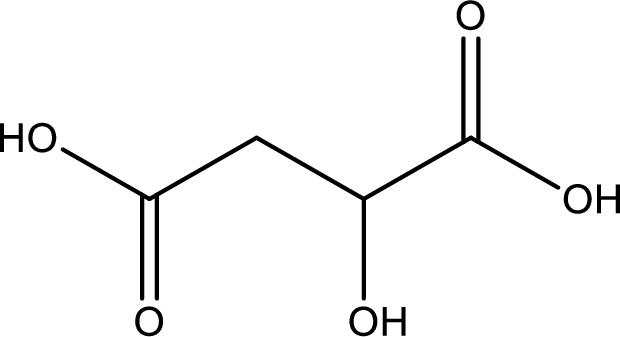
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The parent alkane is butane. The given structure contains two carboxyl groups. The carboxylic acid is named by adding the suffix “-dioic acid”. This gives the name of carboxylic acid as butanedioic acid.
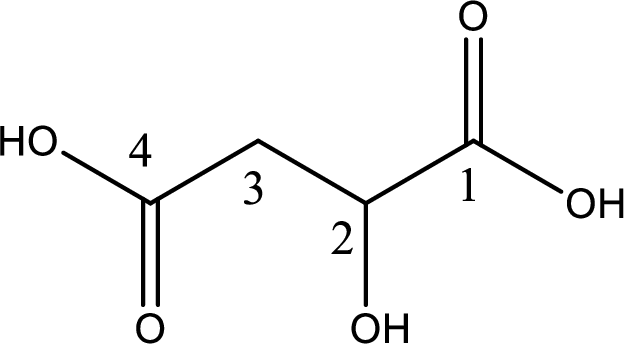
Looking for substituents it is found that there is a hydroxyl group at the second carbon atom. Hence, the IUPAC name of the malic acid is 2-hydroxybutanoic acid.
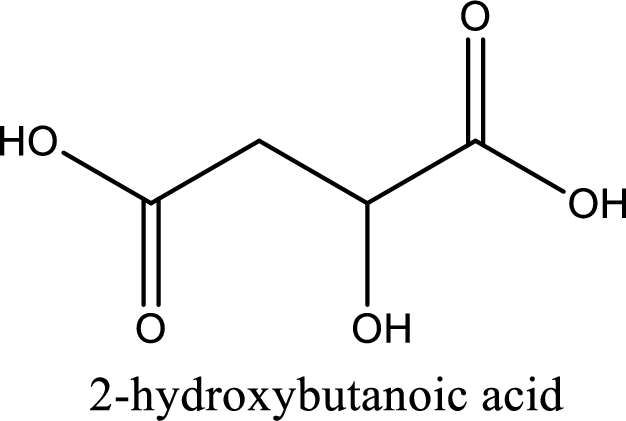
IUPAC name of malic acid is given.
(d)
Interpretation:
IUPAC name for the tartaric acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(d)
Answer to Problem 16.34EP
IUPAC name of tartaric acid is 2,3-dihydroxybutanedioic acid.
Explanation of Solution
Structure of tartaric acid is,
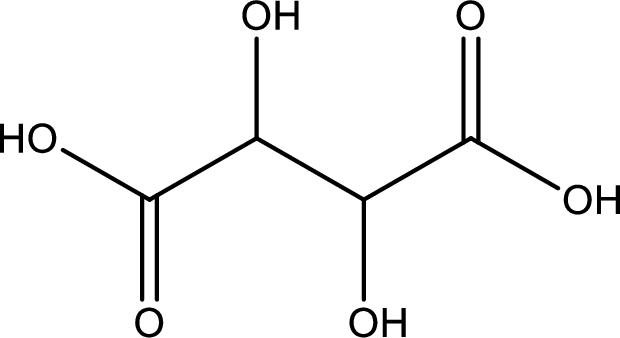
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The parent alkane is butane. The given structure contains two carboxyl groups. The carboxylic acid is named by adding the suffix “-dioic acid”. This gives the name of carboxylic acid as butanedioic acid.
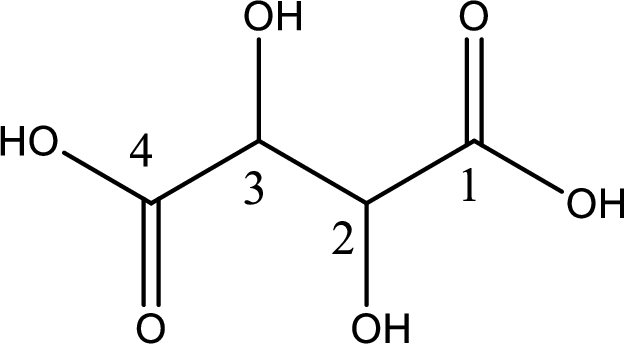
Looking for substituents it is found that there are two hydroxyl groups present, each at the second carbon atom and third carbon atom. Hence, the IUPAC name of the tartaric acid is 2,3-dihydroxybutanoic acid.
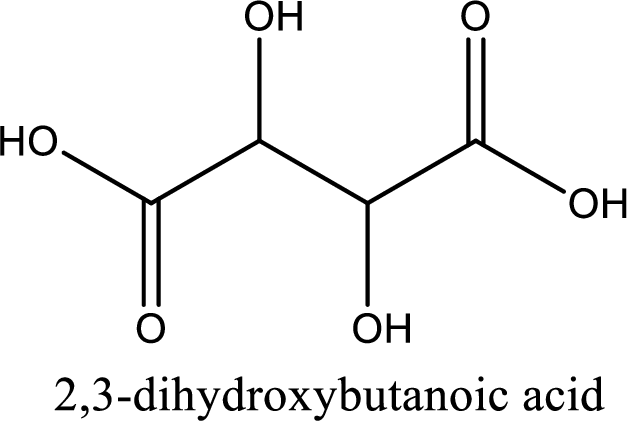
IUPAC name of tartaric acid is given.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- A gas following mole compositions at 120 \deg F, 13.8 psia. N2% 2, CH 4% 79C2H6 % 19. Volume fractionn?arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardOrder-disorder phenomenaa) do not have conductive properties.b) are cooperative.c) have few industrial implications.arrow_forward
- Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





