
Concept explainers
(a)
Interpretation:
Condensed structural formula has to be drawn for the given
Concept Introduction:
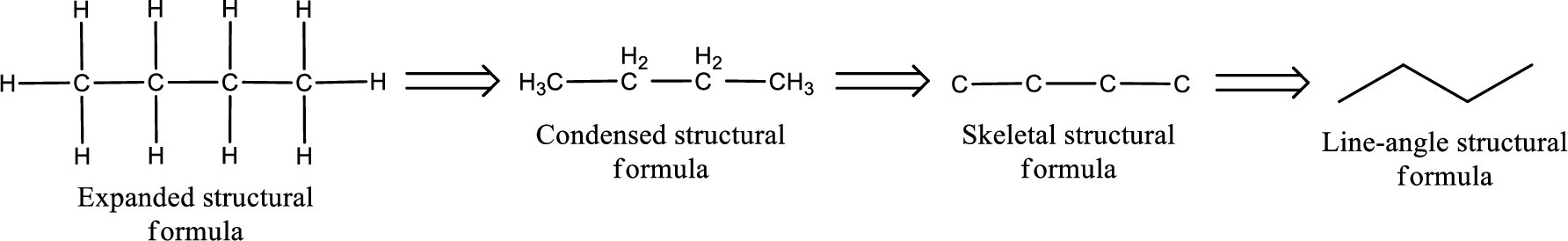
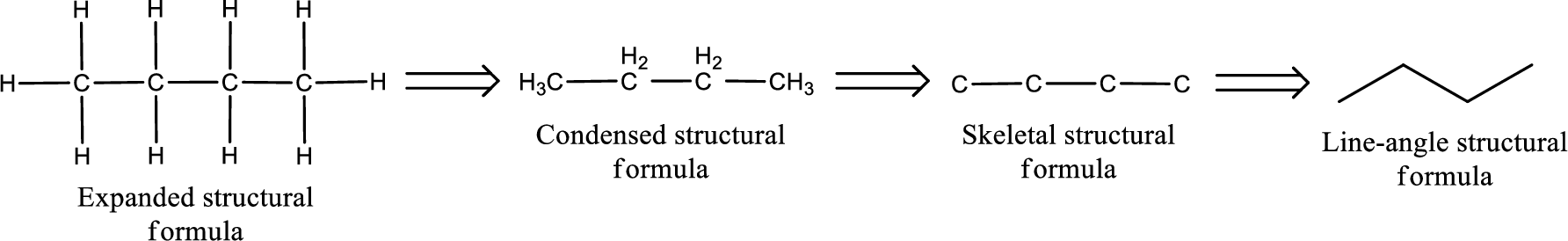
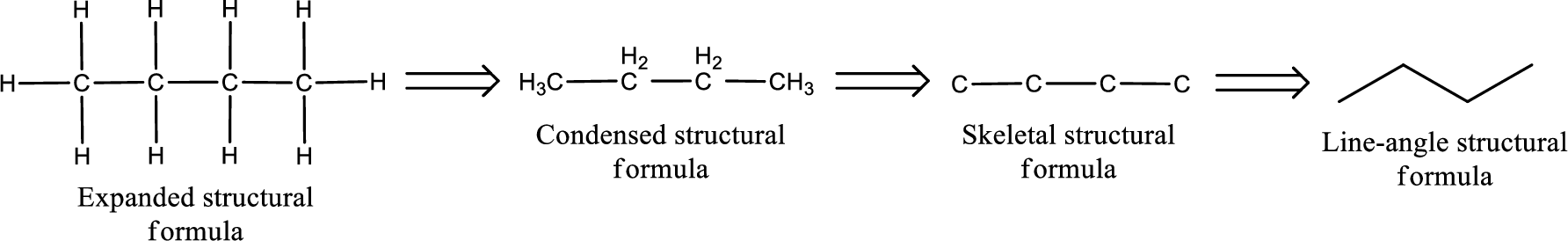
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(a)
Answer to Problem 16.22EP
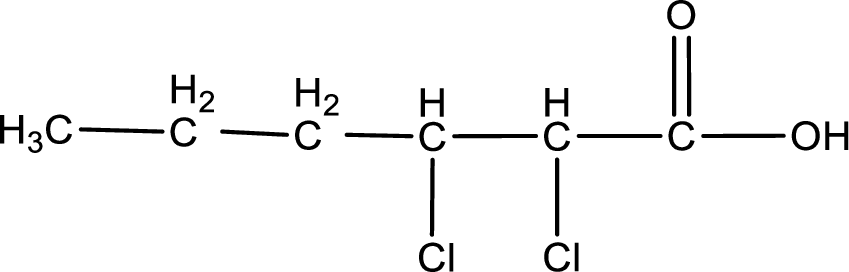
The condensed structural formula is,
Explanation of Solution
Given name of carboxylic acid is 2,3-dichlorohexanoic acid.
From the name it is identified that the parent carbon chain is hexane and the substituent present in the chain are two chlorine atoms, each on second and third carbon atom. The first carbon atom is from the carboxyl group. The structure can be drawn as,
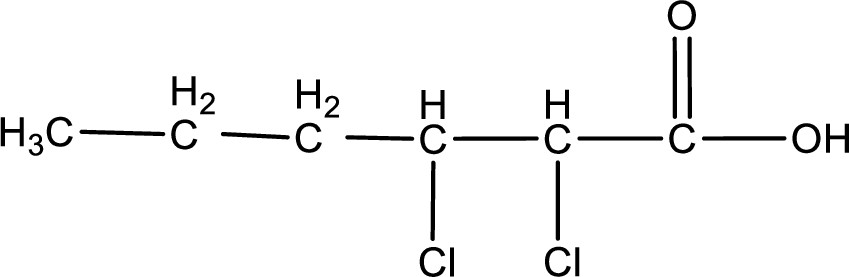
The condensed structural formula for the given carboxylic acid is drawn as shown above.
The condensed structural formula for the given carboxylic acid is drawn.
(b)
Interpretation:
Condensed structural formula has to be drawn for the given carboxylic acid.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(b)
Answer to Problem 16.22EP
The condensed structural formula is,
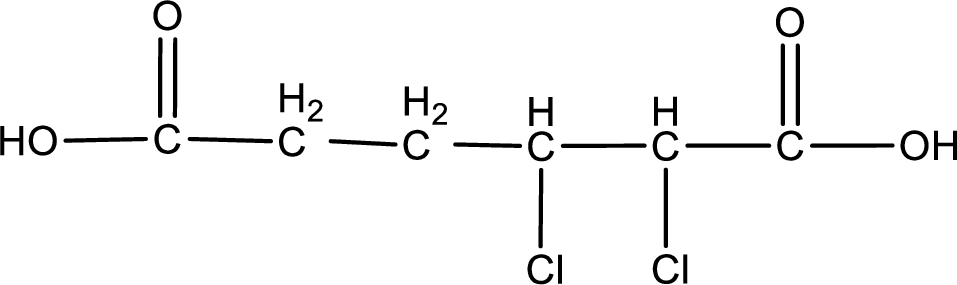
Explanation of Solution
Given name of carboxylic acid is 2,3-dichlorohexanedioic acid.
From the name it is identified that the parent carbon chain is hexane and the substituent present in the chain are two chlorine atoms, each on second and third carbon atom. In the given name suffix “-dioic acid” is present. Therefore, this compound should have two carboxyl groups in it. The first carbon atom and sixth carbon is from the two carboxyl groups. The structure can be drawn as,
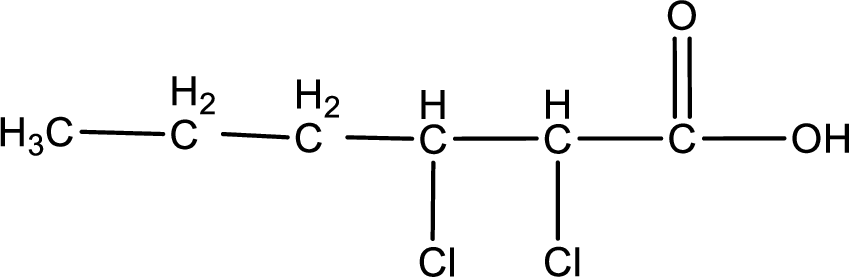
The condensed structural formula for the given carboxylic acid is drawn as shown above.
The condensed structural formula for the given carboxylic acid is drawn.
(c)
Interpretation:
Condensed structural formula has to be drawn for the given carboxylic acid.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(c)
Answer to Problem 16.22EP
The condensed structural formula is,
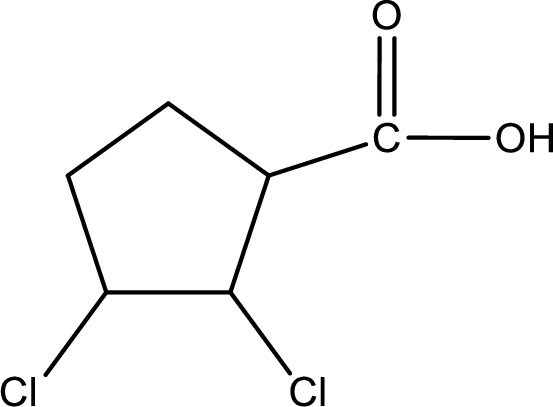
Explanation of Solution
Given name of carboxylic acid is 2,3-dichlorocyclopentanecarboxylic acid.
From the name it is identified that the parent carbon chain is cyclopentane and the substituent present in the chain are two chlorine atoms, each on second carbon atom and third carbon atom. In the given name suffix “-carboxylic acid” is present. Therefore, this compound should have a carboxyl group attached to the cyclopentane ring. The carbon atom in the ring where the carboxyl group is attached is the first carbon atom. The structure can be drawn as,
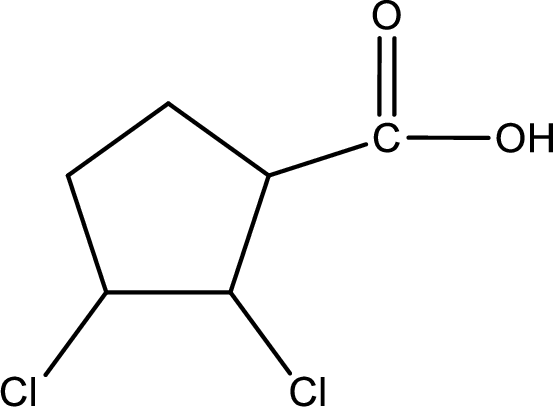
The condensed structural formula for the given carboxylic acid is drawn as shown above.
The condensed structural formula for the given carboxylic acid is drawn.
(d)
Interpretation:
Condensed structural formula has to be drawn for the given carboxylic acid.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(d)
Answer to Problem 16.22EP
The condensed structural formula is,
Explanation of Solution
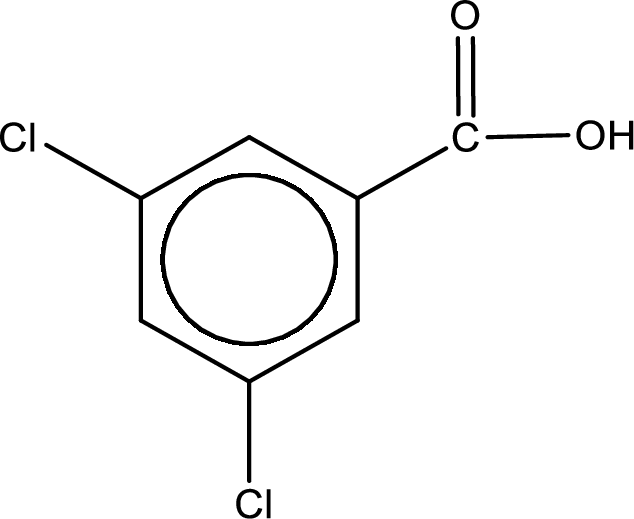
Given name of carboxylic acid is 3,5-dichlorobenzenecarboxylic acid.
From the name it is identified that the parent carbon chain is benzene and the substituent present in the chain are two chlorine atoms, each on third carbon atom and fifth carbon atom. In the given name suffix “-carboxylic acid” is present. Therefore, this compound should have a carboxyl group attached to the benzene ring. The carbon atom in the ring where the carboxyl group is attached is the first carbon atom. The structure can be drawn as,
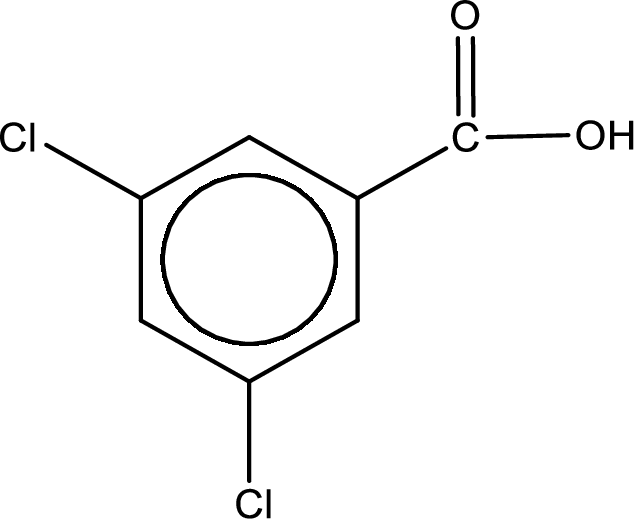
The condensed structural formula for the given carboxylic acid is drawn as shown above.
The condensed structural formula for the given carboxylic acid is drawn.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





