
(a)
Interpretation:
The structure of thioester formed when reaction takes place between
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
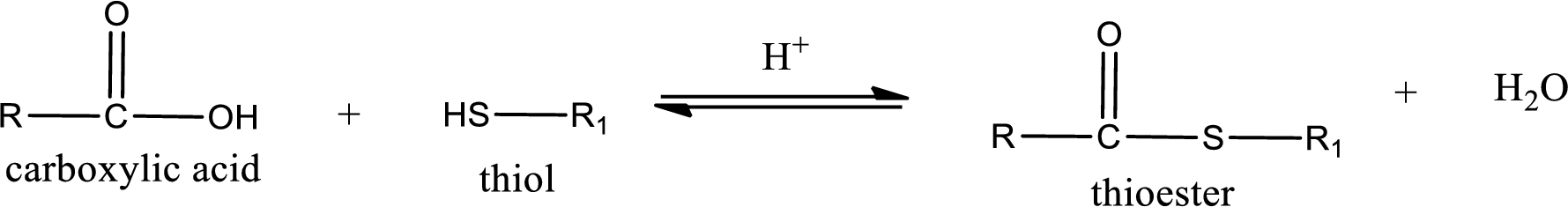
(b)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
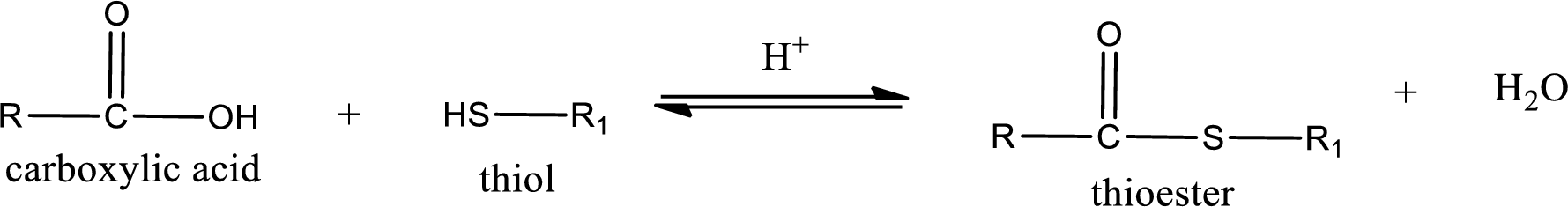
(c)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
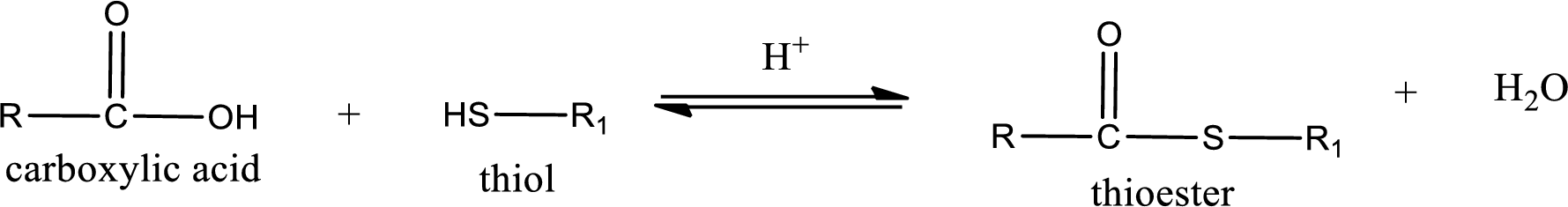
(d)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
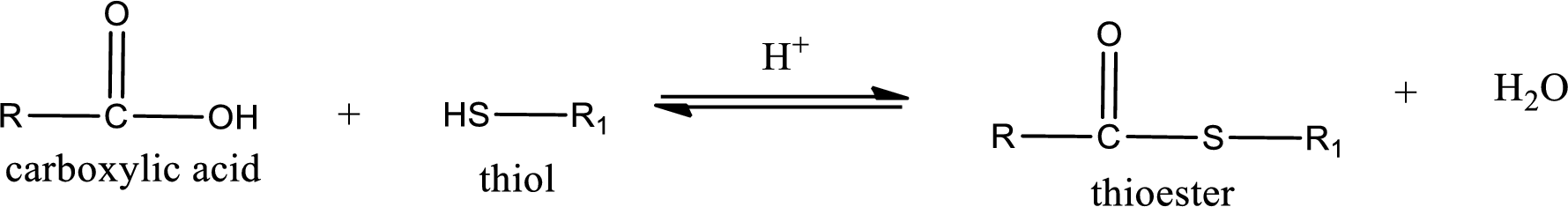
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- Ph heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forward
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


