
Concept explainers
Interpretation:
The compounds in which carboxyl group is present has to be chosen from the given options.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
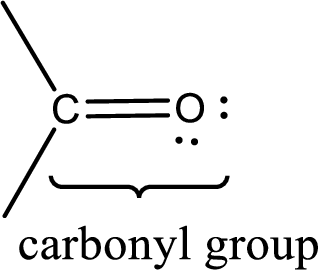
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
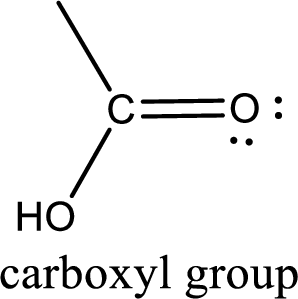
Answer to Problem 16.1EP
Compounds that contain carboxyl groups are option (a) and option (d).
Explanation of Solution
Reason for correct option:
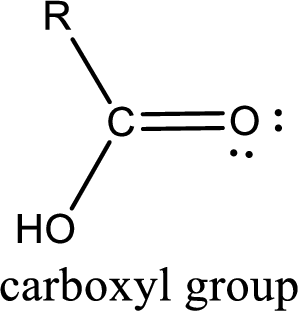
This group is also known as carboxyl group. Among the given compounds in the problem statement, there are only two compounds that contain the carboxyl group. They are shown below,

Hence, the correct options are option (a) and option (d).
Reason for incorrect option:
Compound given in option (b) is,
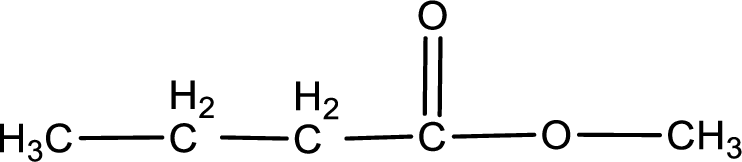
This does not contain the carboxyl group. This is a carboxylic acid derivative namely, ester. Hence, option (b) is an incorrect one.
Compound given in option (c) is,
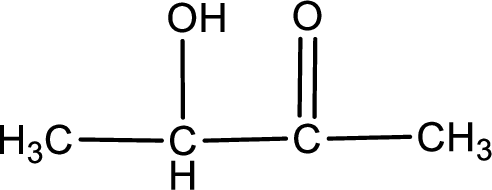
This does not contain the carboxyl group. This is a carbonyl compound. Hence, option (c) is an incorrect one.
Therefore, option (b), and option (c) are incorrect one.
The correct option among the given options is identified as option (a) and option (d).
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





