
Concept explainers
(a)
Interpretation: A name for given compound needs to be assigned.
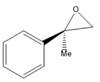
Concept Introduction: Nomenclature of
First method- Parent
Second method: Oxirane ring is considered as parent and groups attached to it are considered as substituents.
(b)
Interpretation: A name for given compound needs to be assigned.
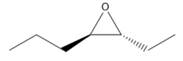
Concept Introduction: Nomenclature of epoxides: Epoxides are cyclic ethers having an oxygen atom incorporated in three-membered ring system having up to four R groups. Due to ring strain, they are more reactive than other ethers. The simplest epoxide does not have any R group and known as ethylene oxide. Naming is done by two methods.
First method- Parent alkane chain is identified and oxygen is considered as substituent on that chain. Location of epoxide group is written with two numbers with suffix epoxy. While naming, epoxy substituents are alphabetically arranged.
Second method: Oxirane ring is considered as parent and groups attached to it are considered as substituents.
(c)
Interpretation: A name for given compound needs to be assigned.
Concept Introduction: Nomenclature of epoxides: Epoxides are cyclic ethers having an oxygen atom incorporated in three-membered ring system having up to four R groups. Due to ring strain, they are more reactive than other ethers. The simplest epoxide does not have any R group and known as ethylene oxide. Naming is done by two methods.
First method- Parent alkane chain is identified and oxygen is considered as substituent on that chain. Location of epoxide group is written with two numbers with suffix epoxy. While naming, epoxy substituents are alphabetically arranged.
Second method: Oxirane ring is considered as parent and groups attached to it are considered as substituents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Which of the following molecules can have enantiomers? Identify any chiral carbon atoms. a. b. c.arrow_forwardWhy are carbon atoms 1 and 3 of glyceraldehyde not considered chiral?arrow_forwardIdentify the configuration of the double bond of the given compound. Identify the chiral centers using an asterisk.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



