
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.8, Problem 14ATS
Interpretation Introduction
Interpretation: For the given reaction, the structure of compound 2 needs to be drawn. Also, reagents used for converting 1 into 3 need to be identified.
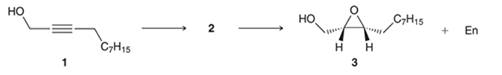
Concept introduction:
Preparation of epoxide-
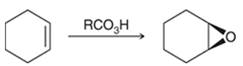
Peroxy acids generally used in this process are MCPBA and peroxyacetic acid. The formation of epoxide via peroxy acid is a stereospecific process; thus, cis substituents in alkene (starting material) remain at cis to each other in the epoxide (product). Similarly, trans substituents in alkene remain at trans to each other.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Grignard reagent is a versatile tool in synthetic organic chemistry. Using bromocyclopentane as a starting material, show how a Grignard reagent, X, is synthesized.
Reaction of X with water produces compound Y while treatment in carbon dioxide followed by hydrolysis forms compound Z. 3-methyl-2butanone reacts with X and hydrolyses to yield compound AA. Draw the structural formulae of compounds Y, Z and AA and write the chemical equations respectively.
Treatment of a hydrocarbon A (molecular formula C9H18) with Br2 in the presence of light forms alkyl halides B and C, both having molecular formula C9H17Br. Reaction of either B or C with KOC(CH3)3 forms compound D (C9H16) as the major product. Ozonolysis of D forms cyclohexanone and acetone. Identify the structures of A–D.
Carototoxin is a natural pesticide produced by carrots, with formula C₁H2O. It undergoes hydrogenation with Pd to:
give product A, C,H,O, and with Lindlar's catalyst to give product B, C,H2O. Ozonolysis followed by zinc leads to a
mixture of methanal, octanal, 1,2-ethanedioic acid, 3-oxopropanoic acid, and 2-hydroxy-3-oxopropanoic acid. Draw
possible structures for carototoxin, A, and B.
Product B structure
H2, Lindlar's
Structure of
carototoxin
H2, Pd/C
Product A structure
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - Prob. 5PTSCh. 13.5 - Prob. 6ATSCh. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 19ATSCh. 13.11 - Prob. 20CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 7LTSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Prob. 33PPCh. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46ASPCh. 13 - Prob. 47ASPCh. 13 - Prob. 48ASPCh. 13 - Prob. 49ASPCh. 13 - Prob. 50ASPCh. 13 - Prob. 51ASPCh. 13 - Prob. 52ASPCh. 13 - Prob. 53ASPCh. 13 - Prob. 54IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - Prob. 71IPCh. 13 - Prob. 72IPCh. 13 - Prob. 73IPCh. 13 - Prob. 74IPCh. 13 - Prob. 77CPCh. 13 - Prob. 79CPCh. 13 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardEnamines normally react with methyl iodide to give two products: one arising from alkylation at nitrogen and the second arising from alkylation at carbon. For example, Heating the mixture of C-alkylation and N-alkylation products gives only the product from C-alkylation. Propose a mechanism for this isomerization.arrow_forward(S)-2-butanol reacts with potassium dichromate (K2CrO4) in aqueous sulfuric acid to give A(C,HgO). Treatment of A with ethylmagnesium bromide in anhydrous ether gives B(C,H140). Draw the structure of B.arrow_forward
- An unknown hydrocarbon A with the formula C6H10 reacts with 1 molar equivalent of H2 over a palladium catalyst to give B C6H12 (Rxn 1). Hydrocarbon A also reacts with OsO4 to give the glycol C (Rxn 2). A gives 5-oxohexanal on ozonolysis (Rxn 3). Draw the structures of A, B, and C. Give the reactions.arrow_forwardCompound AA has a molecular formula of C3H6O and gives a positiveresult using Tollen’s reagent. The reaction of compound AA with hotacidified potassium permanganate, KMnO4 gives compound BB. Thecatalytic hydrogenation of compound AA with nickel, Ni producedcompound CC. The reaction of compound BB with ethanamine,CH3CH2NH2 produces compound DD I) Draw the structural formula of compounds AA, BB, CC and DD. 2)Name the type of chemical reaction for the formation of compound CC.arrow_forward5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forward
- Birch Reduction of toluene leads to a product, X with the molecular formula C7H10. After ozonolysis of X, the two compounds, 3-oxobutanal and malonaldehyde are formed. what is the structure of xarrow_forwardCompound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr) which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (Ch3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2SO4 gives E (C10H16), which reacts with 2 equivalents of Br2 to give F (C10H16Br4). E undergoes hydrogenation with excess H2 and Pt catalyst to give 2-methylpropylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.arrow_forward4. Compound A has the formula C 8H 8. It reacts rapidly with KMnO 4 to give CO 2 and a carboxylic acid, B (C 7H 6O 2), but reacts with only 1 molar equivalent of H 2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H 2 are taken up and hydrocarbon C (C 8H 16) is produced. What are the structures of A, B, and C.arrow_forward
- 10. M and N are amines with the molecular formula C3H»N. Reaction of M with sodium nitrite and HCI releases nitrogen gas and produces a mixture of X and Y and Propanol while N produces a yellowish oily compound, S when reacted with the same reagents. Give the structures of M, N, X, Y, and S. Outline the synthesis of M from a suitable alkene.arrow_forward5b. Enolates have two resonance structures, and can react on oxygen instead of carbon. Show the product of reaction in the oxygen, and then show how it can be converted to the product with the allyl group on carbon.arrow_forwardSharpless epoxidation of allylic alcohol X forms compound Y. Treatment of Y with NaOH and C6H5SH in an alcohol–water mixture forms Z. Identify the structure of Y and draw a mechanism for the conversion of Y to Z. Account for the stereochemistry of the stereogenic centers in Z. Z has been used as an intermediate in the synthesis of chiral carbohydrates.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License