
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 17.6E
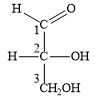
Why are carbon atoms
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Pleasssssseeee solve this question in cheeemsirty, thankss sir
Show work. Don't give Ai generated solution
Pleasssssseeee solve this question in cheeemsirty, thankss sir
Chapter 17 Solutions
Chemistry for Today: General, Organic, and Biochemistry
Ch. 17 - Prob. 17.1ECh. 17 - Describe whether each of the following substances...Ch. 17 - Prob. 17.3ECh. 17 - Prob. 17.4ECh. 17 - Prob. 17.5ECh. 17 - Why are carbon atoms 1 and 3 of glyceraldehyde not...Ch. 17 - Prob. 17.7ECh. 17 - Which of the following molecules can have...Ch. 17 - Which of the following molecules can have...Ch. 17 - Prob. 17.10E
Ch. 17 - Prob. 17.11ECh. 17 - Prob. 17.12ECh. 17 - Prob. 17.13ECh. 17 - Draw Fischer projections for both the D and L...Ch. 17 - Prob. 17.15ECh. 17 - Prob. 17.16ECh. 17 - Prob. 17.17ECh. 17 - Prob. 17.18ECh. 17 - Prob. 17.19ECh. 17 - Prob. 17.20ECh. 17 - Prob. 17.21ECh. 17 - Prob. 17.22ECh. 17 - Prob. 17.23ECh. 17 - Prob. 17.24ECh. 17 - Prob. 17.25ECh. 17 - Prob. 17.26ECh. 17 - Prob. 17.27ECh. 17 - Prob. 17.28ECh. 17 - Prob. 17.29ECh. 17 - Prob. 17.30ECh. 17 - Prob. 17.31ECh. 17 - Prob. 17.32ECh. 17 - Prob. 17.33ECh. 17 - Prob. 17.34ECh. 17 - Prob. 17.35ECh. 17 - Prob. 17.36ECh. 17 - Prob. 17.37ECh. 17 - Prob. 17.38ECh. 17 - Prob. 17.39ECh. 17 - Prob. 17.40ECh. 17 - Prob. 17.41ECh. 17 - Prob. 17.42ECh. 17 - Prob. 17.43ECh. 17 - Prob. 17.44ECh. 17 - Prob. 17.45ECh. 17 - Prob. 17.46ECh. 17 - Prob. 17.47ECh. 17 - Sucrose and honey are commonly used sweeteners....Ch. 17 - Prob. 17.49ECh. 17 - Prob. 17.50ECh. 17 - Prob. 17.51ECh. 17 - Prob. 17.52ECh. 17 - Prob. 17.53ECh. 17 - Prob. 17.54ECh. 17 - Prob. 17.55ECh. 17 - Prob. 17.56ECh. 17 - Prob. 17.57ECh. 17 - Prob. 17.58ECh. 17 - Prob. 17.59ECh. 17 - Prob. 17.60ECh. 17 - Prob. 17.61ECh. 17 - Prob. 17.62ECh. 17 - Prob. 17.63ECh. 17 - Prob. 17.64ECh. 17 - Prob. 17.65ECh. 17 - Prob. 17.66ECh. 17 - Prob. 17.67ECh. 17 - Prob. 17.68ECh. 17 - Prob. 17.69ECh. 17 - Prob. 17.70ECh. 17 - Prob. 17.71ECh. 17 - Prob. 17.72ECh. 17 - Prob. 17.73ECh. 17 - Glucose is a reducing sugar, which if boiled in...Ch. 17 - Prob. 17.75E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Only 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardPart A Give the IUPAC name and a common name for the following ether: CH3-CH2-O-CH2-CH2-CH3 Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Up Part B Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Uparrow_forwardFrenkel and Schottky are intrinsic or extrinsic defects, point or linear defects.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY