
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 72IP
Interpretation Introduction
Interpretation: The synthesis of
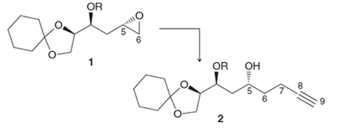
Concept Introduction:
In the acid-catalyzed ring-opening of an epoxide with water, first proton transfer takes place and then nucleophilic attack takes place via the SN2 mechanism. In the end, proton transfer step takes place that removes the charge formed after the attack of the neutral nucleophile on a more substituted position. Similarly, ring opening of epoxide can also take place in a basic medium. This results in an attack of the nucleophile on a less substituted position.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Treatment of a hydrocarbon A (molecular formula C9H18) with Br2 in the presence of light forms alkyl halides B and C, both having molecular formula C9H17Br. Reaction of either B or C with KOC(CH3)3 forms compound D (C9H16) as the major product. Ozonolysis of D forms cyclohexanone and acetone. Identify the structures of A–D.
3. An alcohol containing natural product A (C10H200) that is a common ingredient in cough drops
yields two structurally isomeric alkenes, 1 and 2, on dehydration. Ozonolysis followed by an
oxidative workup or a reductive workup as shown yields 3 and 4 from 1 and 2, respectively.
Hydroboration of alkene 1 yields the starting alcohol A as the major product from 1. Hydroboration
yields a mixture of the starting alcohol A and a diastereomer of A starting from alkene 2. Given this
information deduce the structure of A, 1, and 2. Show the correct absolute and relative
stereochemistry for A (the alcohol group is cis to a methyl group), 1, and 2 and correctly name these
three compounds.
H2SO4
heat
1) O3
Н.
1 (C10H18)
2. Zn, H3O*
3
H.
A (C10H200)
H2SO4
1) O3
н СНО
heat
2 (C10H18)
2. Zn, H3O*
Н 4
Treatment of anthranilic acid with nitrous acid gives an intermediate, A, that contains a
diazonium ion and a carboxylate group. When this intermediate is heated in the pres-
ence of furan, a tricyclic compound is formed. Propose a structural formula for com-
pound A and a mechanism for the formation of the tricyclic product.
COOH
NANO, HCI
+ CO, + N2
NH2
Anthranilic
acid
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - Prob. 5PTSCh. 13.5 - Prob. 6ATSCh. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 19ATSCh. 13.11 - Prob. 20CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 7LTSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Prob. 33PPCh. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46ASPCh. 13 - Prob. 47ASPCh. 13 - Prob. 48ASPCh. 13 - Prob. 49ASPCh. 13 - Prob. 50ASPCh. 13 - Prob. 51ASPCh. 13 - Prob. 52ASPCh. 13 - Prob. 53ASPCh. 13 - Prob. 54IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - Prob. 71IPCh. 13 - Prob. 72IPCh. 13 - Prob. 73IPCh. 13 - Prob. 74IPCh. 13 - Prob. 77CPCh. 13 - Prob. 79CPCh. 13 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardWhen warmed in dilute sulfuric acid, 1-phenyl-1,2-propanediol undergoes dehydration and rearrangement to give 2-phenylpropanal. (a) Propose a mechanism for this example of a pinacol rearrangement (Section 10.7). (b) Account for the fact that 2-phenylpropanal is formed rather than its constitutional isomer, 1-phenyl-1-propanone.arrow_forwardAlcohols are important for organic synthesis, especially in situations involving alkenes. The alcohol might be the desired product, or the OH group might be transformed into another functional group via halogenation, oxidation, or perhaps conversion to a sulfonic ester derivative. Formation of an alcohol from an alkene is particularly powerful because conditions can be chosen to produce either the Markovnikov or non-Markovnikov product from an unsymmetrical alkene. Using your reaction roadmap as a guide, show how to convert 4-methyl-1-pentene into 5-methylhexanenitrile. You must use 4-methyl-1-pentene and sodium cyanide as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way.arrow_forward
- A newer generation of antipsychotics, among them clozapine, are now used to treat the symptoms of schizophrenia. These drugs are more effective than earlier drugs in improving patient response in the areas of social withdrawal, apathy, memory, comprehension, and judgment. They also produce fewer side effects such as seizures and tardive dyskinesia (involuntary body movements). In the following synthesis of clozapine, Step 1 is an Ullmann coupling, a type of nucleophilic aromatic substitution that uses a copper catalyst. (a) Show how you might bring about formation of the amide in Step 2. (b) Propose a reagent for Step 3. (c) Propose a mechanism for Step 4. (d) Is clozapine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardEnamines normally react with methyl iodide to give two products: one arising from alkylation at nitrogen and the second arising from alkylation at carbon. For example, Heating the mixture of C-alkylation and N-alkylation products gives only the product from C-alkylation. Propose a mechanism for this isomerization.arrow_forwardBicyclo-2,5-heptadiene can be prepared in two steps from cyclopentadiene and vinyl chloride. Provide a mechanism for each step.arrow_forward
- Heck reactions take place with alkynes as well as alkenes. The following conversion involves an intramolecular Heck reaction followed by an intermolecular Heck. Propose structural formulas for the palladium-containing intermediates involved in this reaction.arrow_forwardAnother important pattern in organic synthesis is the construction of CC bonds. Using your reaction roadmap as a guide, show how to convert propane into hex-1-en-4-yne. You must use propane as the source of all of the carbon atoms in the hex-1-en-4-yne product. Show all reagents needed and all molecules synthesized along the way.arrow_forwardThree isomeric pentanols with unbranched carbon chains exist. Which of these isomers, upon dehydration at 180C, yields only 1-pentene as a product?arrow_forward
- H3C N- H₂NNH₂ H⭑ CH3 H3C IN CH3 Hydrazine reacts with 2,4-pentanedione to yield 3,5-dimethylpyrazole. Including protonations and deprotonations, the reaction takes 12 steps. Write out the mechanism on a sheet of paper and then draw the structure of the product of step 6. • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • In an elimination step, include the structure of the leaving group, but draw it in its own sketcher. Separate structures with + signs from the drop-down menu. ? Sn th Previous Nextarrow_forwardThe sex attractant of the female winter moth has been identified as the tetraene CH3(CH2)8CH=CHCH2CH=CHCH2CH=CHCH=CH2. Devise a synthesis of this material from 3,6-hexadecadien-1-ol and allyl alcohol.arrow_forwardNatural products that contain the N-1,1-dimethyl-2-propenyl group often exhibit antifungal or antitumor activity. The synthesis of one such compound begins with the two steps shown here. OAc C13H15N CuCl, -Pr2NEt 2 3 a. Draw the structure of compound 2 and show a reasonable mechanism for its formation. CuCl is used as a catalyst (behaving similarly to an acid) and i-Pr2NEt is a base. The former can be ignored in your mechanism. b. Identify the reagents necessary for the conversion of 2 to 3.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY