
Concept explainers
(a)
Interpretation:
The reagents used for the synthesis of
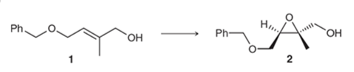
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to an allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(b)
Interpretation:
The mechanism for the preparation of orthoester 4 from epoxide 3 needs to be proposed.
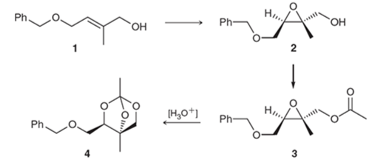
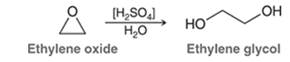
Concept Introduction: In the acid-catalyzed ring-opening of an epoxide with water, first proton transfer takes place and then nucleophilic attack takes place via an SN2 mechanism. In the end, proton transfer step takes place that removes the charge formed after the attack of the neutral nucleophile. The general reaction is represented as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Following is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward5 Propose a reaction for the preparation of benzopinacol from benzophenone that does not involve photochemistry. Include the mechanism for the reaction.arrow_forwardGive the major organic product of each reaction of γ‑valerolactone with each of the given six reagents under the conditions indicated. Do not draw any byproducts formed. Reagent 1. NaOH, H2O, heat, then H+, H2O 2. (CH3)2CHCH2CH2OH (excess), H+ 3. (CH3CH2)2NH and heat 4. CH3MgI (excess), ether, then H+/H2O 5. LiAlH4, ether, then H+/H2O 6. DIBAL, toluene, low temperature, then H+/H2Oarrow_forward
- Compelling evidence for the existence of a tetrahedral intermediate in nucleophilic acyl substitution was obtained in a series of elegant experiments carried out by Myron Bender in 1951. The key experiment was the reaction of aqueous−OH with ethyl benzoate (C6H5COOCH2CH3) labeled at the carbonyl oxygen with 18O. Bender did not allow the hydrolysis to go to completion, and then examined the presence of a label in the recovered starting material. He found that some of the recovered ethyl benzoate no longer contained a label at the carbonyl oxygen. With reference to the accepted mechanism of nucleophllic acyl substitution, explain how this provides evidence for a tetrahedral intermediate.arrow_forwardThe widely used anticoagulant warfarin (see "Chemical Connections: From Moldy Clover to a Blood Thinner" in Chapter 18) is synthesized from 4-hydroxycoumarin, benzaldehyde, and acetone as shown in this retrosynthesis. Show how warfarin is synthesized from these reagents. OH OH Acetone + Warfarin 4Нydroxy- (a synthetic anticoagulant; racemic) coumarin Benzaldehydearrow_forward11. Give a structure for the compounds 1), 2) and 3) in the following synthesis. 1) 2) 1) LiAlH4 2) H3O+ 3) H₂SO4/A 3)arrow_forward
- Compelling evidence for the existence of a tetrahedral intermediate innucleophilic acyl substitution was obtained in a series of elegantexperiments carried out by Myron Bender in 1951. The key experimentwas the reaction of aqueous −OH with ethyl benzoate (C6H5COOCH2CH3)labeled at the carbonyl oxygen with 18O. Bender did not allow thehydrolysis to go to completion, and then examined the presence of alabel in the recovered starting material. He found that some of therecovered ethyl benzoate no longer contained a label at the carbonyloxygen. With reference to the accepted mechanism of nucleophilic acyl substitution, explain how this provides evidence for a tetrahedralintermediate.arrow_forward5. Optically pure 2-octyl sulfonate was treated with varying mixtures of water and dioxane, and the optical purity of the resulting product (2-octanol) was found to vary with the ratio of water to dioxane, as shown in the following table (J. Am. Chem. Soc. 1965, 87, 287-291). Given that dioxane possesses fairly nucleophilic oxygen atoms, provide a complete mechanism that explains the variation in in the product's optical purity due to changes in solvent composition. Hint, the solvent is doing more here than just dissolving.. it is partaking in the reaction. Solvent Ratio (water : dioxane) 25:75 50: 50 75: 25 100 :0 Optical purity of (S)-2-octanol 77% 88% 95% 100% H20 он (R)-2-Octyl sulfonate (optically pure) (S)-2-Octanol (dioxane)arrow_forwardDoxaprost, an orally active bronchodilator patterned after the natural prostaglandins , is synthesized in the following series of reactions starting with ethyl 2-oxocyclopentanecarboxylate. Except for the Nef reaction in Step 8, we have seen examples of all other types of reactions involved in this synthesis. Q. Describe experimental conditions to bring about Step 7 and account for the fact that the trans isomer is formed in this step.arrow_forward
- A convenient method for achieving the transformation below involves treatment of the ketone with Wittig reagent 1 followed by acid- catalyzed hydrolysis (Chem. Ber. 1962, 95, 2514-2525): 1) MeO 2) H₂O+ Show Transcribed Text =PPh3 or H Draw the mechanisms of the following reaction.arrow_forward8. Using only ethylacetate, acetone, benzaldehyde, or benzyl bromide as organic components, and using any bases you desire, indicate a method to synthesize the following compounds: 요요 ilarrow_forward(b) Provide retrosynthetic steps leading to ketone 9 from bicyclic compound 8. Provide all intermediates (synthons) and their equivalents clearly labelled. No synthetic steps and mechanisms are required. 8-1 NC. 8 9arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
