
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 86P
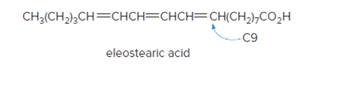
Eleostearic acid is an unsaturated fatty acid found in tung oil, obtained from the seeds of the tung oil tree (Aleurites for dii) a deciduous tree native to China. Eleostearic acid is unusual in that the double bond at C9 is cis, but the other two double bonds are trans, (a) Draw the structure of eleostearic acid, showing the arrangement of groups around each double bond, (b) Draw a stereoisomer of eleostearic acid in which all of the double bonds are trans, (c) Which compound, eleostearic acid or its all-trans isomer, has the higher melting point? Explain your reasoning.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the skeletal structure of the
alkane 4-ethyl-2, 2, 5, 5-
tetramethylnonane. How many
primary, secondary, tertiary, and
quantenary carbons does it have?
Don't used Ai solution
Don't used Ai solution
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 13.1 - Convert each condensed structure to a complete...Ch. 13.1 - Prob. 13.1PCh. 13.1 - Complete the structure of zingiberene, a component...Ch. 13.2 - Prob. 13.2PPCh. 13.2 - Prob. 13.3PCh. 13.2 - Prob. 13.3PPCh. 13.2 - Prob. 13.4PPCh. 13.3 - Prob. 13.5PPCh. 13.3 - Prob. 13.4PCh. 13.3 - Prob. 13.6PP
Ch. 13.3 - Prob. 13.5PCh. 13.3 - Prob. 13.6PCh. 13.3 - Prob. 13.7PCh. 13.3 - Prob. 13.8PCh. 13.6 - Prob. 13.7PPCh. 13.6 - Prob. 13.9PCh. 13.6 - What products is formed in each of the following...Ch. 13.6 - Prob. 13.9PPCh. 13.6 - What product is formed when l-pentene (CH3CH2CH2CH...Ch. 13.7 - Prob. 13.11PCh. 13.8 - Prob. 13.10PPCh. 13.8 - Prob. 13.12PCh. 13.8 - Prob. 13.13PCh. 13.10 - Prob. 13.11PPCh. 13.10 - Prob. 13.14PCh. 13.11 - Prob. 13.15PCh. 13.12 - Prob. 13.16PCh. 13.13 - Prob. 13.17PCh. 13.13 - Prob. 13.18PCh. 13 - Anethole, the major constituent of anise oil, is...Ch. 13 - Prob. 20PCh. 13 - What is the molecular formula for a hydrocarbon...Ch. 13 - Prob. 22PCh. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - Prob. 26PCh. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each compound. a....Ch. 13 - Give the IUPAC name for each compound. d....Ch. 13 - Give the IUPAC name for each alkene.Ch. 13 - Give the I UP AC name for each alkene.Ch. 13 - Give the IUPAC name for each cyclic compound.Ch. 13 - Give the IUPAC name for each cyclic compound.Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 36PCh. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Label the carbon-carbon double bond as cis or...Ch. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - What alkyd halide is formed when each alkene is...Ch. 13 - Prob. 56PCh. 13 - Prob. 57PCh. 13 - Prob. 58PCh. 13 - What alkene is needed as a starting material to...Ch. 13 - Prob. 60PCh. 13 - Prob. 61PCh. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - Prob. 64PCh. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Prob. 67PCh. 13 - Prob. 68PCh. 13 - Prob. 69PCh. 13 - Prob. 70PCh. 13 - Prob. 71PCh. 13 - Prob. 72PCh. 13 - Prob. 73PCh. 13 - Are o-bromochlorobenzene and m-bromochlorobenzene...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 77PCh. 13 - Prob. 78PCh. 13 - Prob. 79PCh. 13 - Prob. 80PCh. 13 - Prob. 81PCh. 13 - Prob. 82PCh. 13 - Prob. 83PCh. 13 - Prob. 84PCh. 13 - Prob. 85PCh. 13 - Eleostearic acid is an unsaturated fatty acid...Ch. 13 - Prob. 87PCh. 13 - Prob. 88PCh. 13 - Prob. 89PCh. 13 - Prob. 90PCh. 13 - Prob. 91PCh. 13 - Prob. 92PCh. 13 - Prob. 93PCh. 13 - Prob. 94PCh. 13 - Answer the following questions about compound A,...Ch. 13 - Prob. 96PCh. 13 - Answer the following questions about alkene C,...Ch. 13 - Prob. 98PCh. 13 - Prob. 99PCh. 13 - Prob. 100PCh. 13 - Prob. 101CPCh. 13 - Prob. 102CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY