
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 13.73AP
a.
Interpretation Introduction
Interpretation:
Product of the acid-base reaction has to be drawn.
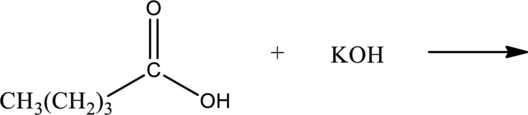
Concept Introduction:
Water soluble salts are obtained as products when a
b.
Interpretation Introduction
Interpretation:
Product of the acid-base reaction has to be drawn.

Concept Introduction:
Refer part “a.”.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
b) Certain cyclic compounds are known to be conformationally similar to carbohydrates, although they are not
themselves carbohydrates. One example is Compound C shown below, which could be imagined as adopting
four possible conformations. In reality, however, only one of these is particularly stable. Circle the conformation
you expect to be the most stable, and provide an explanation to justify your choice. For your explanation to be
both convincing and correct, it must contain not only words, but also "cartoon" orbital drawings contrasting the
four structures.
Compound C
Possible conformations (circle one):
Дет
Lab Data
The distance entered is out of the expected range.
Check your calculations and conversion factors.
Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3?
Did you report your data to the correct number of significant figures?
- X
Experimental Set-up
HCI-NH3
NH3-HCI
Longer Tube
Time elapsed (min)
5 (exact)
5 (exact)
Distance between cotton balls (cm)
24.30
24.40
Distance to cloud (cm)
9.70
14.16
Distance traveled by HCI (cm)
9.70
9.80
Distance traveled by NH3 (cm)
14.60
14.50
Diffusion rate of HCI (cm/hr)
116
118
Diffusion rate of NH3 (cm/hr)
175.2
175.2
How to measure distance and calculate rate
For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically:
1:1 (one mole of EDTA per mole of metal ion)
2:1 (two moles of EDTA per mole of metal ion)
1:2 (one mole of EDTA per two moles of metal ion)
None of the above
Chapter 13 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 13.1 - Draw out each compound to clearly show what groups...Ch. 13.1 - Prob. 13.2PCh. 13.2 - Prob. 13.3PCh. 13.2 - Give the structure corresponding to each IUPAC...Ch. 13.2 - Prob. 13.5PCh. 13.2 - Give the structure corresponding to each name. a....Ch. 13.3 - Which compound in each pair has the higher boiling...Ch. 13.3 - Rank the following compounds in order of...Ch. 13.4 - Which compounds are -hydroxy acids? tartaric acid...Ch. 13.4 - Prob. 13.10P
Ch. 13.5 - Prob. 13.11PCh. 13.5 - Prob. 13.12PCh. 13.5 - Prob. 13.13PCh. 13.6 - Prob. 13.14PCh. 13.6 - Prob. 13.15PCh. 13.6 - Prob. 13.16PCh. 13.6 - Prob. 13.17PCh. 13.6 - Prob. 13.18PCh. 13.6 - Prob. 13.19PCh. 13.7 - Prob. 13.20PCh. 13.7 - Prob. 13.21PCh. 13.7 - Prob. 13.22PCh. 13.7 - Prob. 13.23PCh. 13.7 - Prob. 13.24PCh. 13.8 - Prob. 13.25PCh. 13.8 - Prob. 13.26PCh. 13.8 - Prob. 13.27PCh. 13.8 - Draw the product formed when each ammonium salt is...Ch. 13.8 - Prob. 13.29PCh. 13.9 - Prob. 13.30PCh. 13.9 - Prob. 13.31PCh. 13.9 - Prob. 13.32PCh. 13.9 - Why is the boiling point of CH3CONH2(221C) higher...Ch. 13.9 - Prob. 13.34PCh. 13.9 - Prob. 13.35PCh. 13.10 - Prob. 13.36PCh. 13 - Prob. 13.37UKCCh. 13 - Prob. 13.38UKCCh. 13 - Prob. 13.39UKCCh. 13 - Prob. 13.40UKCCh. 13 - Prob. 13.41UKCCh. 13 - Prob. 13.42UKCCh. 13 - Prob. 13.43UKCCh. 13 - Prob. 13.44UKCCh. 13 - Prob. 13.45UKCCh. 13 - Prob. 13.46UKCCh. 13 - Prob. 13.47UKCCh. 13 - Prob. 13.48UKCCh. 13 - Prob. 13.49UKCCh. 13 - Prob. 13.50UKCCh. 13 - Prob. 13.51APCh. 13 - Prob. 13.52APCh. 13 - Prob. 13.53APCh. 13 - Draw the structure of a compound of molecular...Ch. 13 - Prob. 13.55APCh. 13 - Prob. 13.56APCh. 13 - Give an acceptable name for each compound.Ch. 13 - Prob. 13.58APCh. 13 - Prob. 13.59APCh. 13 - Prob. 13.60APCh. 13 - Prob. 13.61APCh. 13 - Prob. 13.62APCh. 13 - Prob. 13.63APCh. 13 - Give an acceptable name for each amine or amide....Ch. 13 - Draw the structure corresponding to each name. a....Ch. 13 - Draw the structure corresponding to each name. a....Ch. 13 - Prob. 13.67APCh. 13 - Draw the structure of each amine or ammonium salt....Ch. 13 - Prob. 13.69APCh. 13 - Which compound in each pair is more water soluble?...Ch. 13 - Prob. 13.71APCh. 13 - Prob. 13.72APCh. 13 - Prob. 13.73APCh. 13 - Prob. 13.74APCh. 13 - Prob. 13.75APCh. 13 - Prob. 13.76APCh. 13 - Prob. 13.77APCh. 13 - Prob. 13.78APCh. 13 - Prob. 13.79APCh. 13 - Prob. 13.80APCh. 13 - Prob. 13.81APCh. 13 - Prob. 13.82APCh. 13 - Prob. 13.83APCh. 13 - Prob. 13.84APCh. 13 - Prob. 13.85APCh. 13 - Prob. 13.86APCh. 13 - Prob. 13.87APCh. 13 - Draw the products of each acid-base reaction.Ch. 13 - Prob. 13.89APCh. 13 - Prob. 13.90APCh. 13 - Prob. 13.91APCh. 13 - Prob. 13.92APCh. 13 - Ritalin is the trade name for methylphenidate, a...Ch. 13 - Prob. 13.94APCh. 13 - Prob. 13.95CPCh. 13 - Prob. 13.96CPCh. 13 - Prob. 13.97CPCh. 13 - Prob. 13.98BTCCh. 13 - Prob. 13.99BTCCh. 13 - Prob. 13.100BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY