
Concept explainers
(a)
Interpretation:
It should be determined that the product obtained from the reaction of Ethyl butanoate with
Concept introduction:
Reduction of carbonyl, carboxylic acid and ester using
Reduction of an ester (Methyl-2-pentenoate) by using
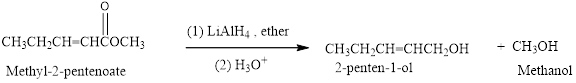
(b)
Interpretation:
It should be determined that the product obtained from the reaction of Methyl benzoate with
Concept introduction:
Reduction of carbonyl, carboxylic acid and ester using
Reduction of an ester (Methyl-2-pentenoate) by using
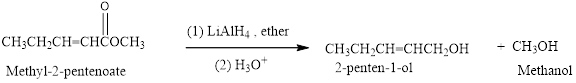
(c)
Interpretation:
It should be determined that the product obtained from the reaction of Pentatonic acid
with
Concept introduction:
Reduction of carbonyl, carboxylic acid and ester using
Reduction of a carboxylic acid (oleic acid) by using
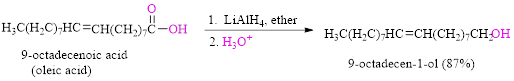
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
- What reagents would you use to convert methyl propanoate to the following compounds? a. isopropyl propanoate b. sodium propanoate c. N-ethylpropanamide d. propanoic acidarrow_forwardExplain the experimental procedure of the laboratory preparation of the synthesis of benzyl chloride from phenol Explain the chemistry behind the synthesis of benzyl chloride from phenolarrow_forwardWhat is the reaction mechanism for formaldehyde and phenol under acidic conditions?arrow_forward
- Give the sequence of the relative reactivities of carboxylic acid derivatives. Explain why acyl chloride can be converted into ester but ester cannot be converted into acyl chloride?arrow_forwardWhat products are formed when the following compounds react with ozone and then with dimethyl sulfide?arrow_forwardWhich of the following reactions will result in the formation of an acyl halide? Select one: a. The reaction of a carboxylic acid with phosphorus trichloride. b. The treatment of an alcohol with ethyl bromide. c. The reaction of an ester with hydrochloric acid. d. The addition of an alkene to dilute hydrochloric acid.arrow_forward
- Why can’t 2-methyl-2-propanol be prepared by the reduction of a carbonyl compound?arrow_forwardAccount for the fact that treating propenoic acid (acrylic acid) with HCl gives only 3-chloropropanoic acid.arrow_forwardIn the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other reagents. Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid. (a) Why are triphenylmethyl ethers so readily hydrolyzed by aqueous acid? (b) How might the structure of the triphenylmethyl group be modified to increase or decrease its acid sensitivity?arrow_forward
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardGive the products formed when benzaldehyde and benzoic acid are treated with the given reagents. 1 mole CH3OH, H+ LiAlH4 then H2O, H+arrow_forwardWhat products are formed when the following compound reacts with ozone and then with dimethyl sulfide?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


