
Concept explainers
(a)
Interpretation: The products for the given reaction have to be found.
Concept Introduction:
Ketals:
Ketals are used to protect the carbonyl group of the
In the following example, the carbonyl group of the acetone has been protected as ketal by using ethylene glycol.
Example:
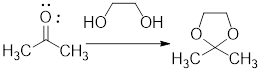
In acidic condition, a ketone reacts with two molecules of alcohol or a molecule of
General scheme:
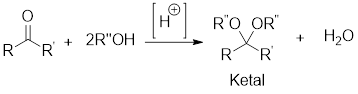
(b)
Interpretation: The products for the given reaction have to be found.
Concept Introduction:
Acetals:
Acetals are used to protect the carbonyl group of the
In the following example, the carbonyl group of the acetaldehyde has been protected as acetal by using ethylene glycol.
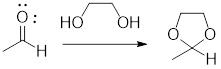
In acidic condition, an aldehyde reacts with two molecules of alcohol or a molecule of diol to form acetal or cyclic acetal respectively.
General scheme:
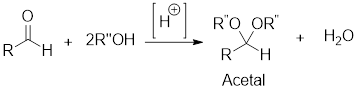
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
- Show how to prepare each compound from 2-methyl- 1- propanol. a. 2- methylpropene b. 2- methyl- 2- propanol c. 2- methylpropanoic acid (CH3)2CHCOOHarrow_forward2. Provide the missing product or starting materials for the following reactions. a. b. C. 1. LDA 2. Xh H 3. H3O+ NaOH heat NaOH OHarrow_forwardGive the products formed when benzaldehyde and benzoic acid are treated with the given reagents. a. Tollen’s reagentb. phenylhydrazine, H+c. HCNd. NH2OHe. 1 mole H2, Nif. 1 mole CH3OH, H+g. LiAlH4 then H2O, H+h. 2 moles CH3OH, H+i. CH3MgCl, then H2O, H+j. H2Oarrow_forward
- Reagents a. C6H5CHO b. NaOH, ethanol h. BrCH2CH=CH2 i. Na* OEt, ethanol j. Br2, H* k. K* t-BuO c. Pyrrolidine, cat. H* d. H2C=CHCN e. H3O* f. I. CH2(CO2ET)2 -CH2CH2CN LDA m. heat g. ELOC(=0)CO2ET Select reagents from the table to synthesize this compound from cyclopentanone. Enter the letters of the chosen reagents, in the order that you wish to use them, without spaces or punctuation (i.e. geda).arrow_forwardWhat is the product of the following reaction? A. B. شما NH NH2Et, cat H* C. ZI avant HO. D. `N OHarrow_forward4. What is the product of the following reaction? A. B. OH 1) CH3CH₂CH3MgBr 2) H₂O C. HO D.arrow_forward
- он C-H ÓR Compound that contains a group like that shown above. a. acetal Ob. aldehyde c. Benedict's reagent d. carbonyl Oe. hemiacetal Of. hemiketal Og. hydrolysis h. ketal i. ketone Oj. Tollen's reagent 2.arrow_forwardWhat product(s) will form: 2-Pentene + H₂O → O a. 2-Pentanol O b. Isopropyl alcohol O c. 3-Pentanol O d. a 50/50 mixture of 2-Pentanol and 3-Pentanol Oe. no reaction will occurarrow_forwardWhat is the major organic product obtained from the following reaction? a. 1 b.2 c. 3 d. 4 -CC-H 1. BH3 2. H₂O₂, NaOH 1 3 OH 2 4 CH₂arrow_forward
- 2-Methyl-2-butene reacts with HBr in the presence of peroxide to give a. a secondary alkyl bromide. b. a primary alkyl bromide. c. a tertiary alkyl bromide. d. a vicinal dibromide.arrow_forward1. An alkene reacts with water with an acid catalyst results into a formation of: A. Aldehyde B. Ketone C. Alcohol D. Ester 2. 3-Methylhexanal with K2Cr2O7 will yield: A. 3-Methyl-1-hexanol B. 3-Methylhexanoic acid C. 3-Methyl-1-hexanone D. 3-Methyl-1-hexanethiol 3. This is a reverse process of Hydration reaction: A. Oxidation reaction B. Reduction reaction C. Dehydration reaction D. Hydration reaction 4. Acetic acid reacts with a strong base forms: A. Salt B. Water C. Salt and Water D. No reaction 5. Ketones can be further oxidized with benedict's solution into: A. Alcohol B. Aldehyde C. Catalysts D. No reactionarrow_forwardWhat is the major organic product obtained from the following reaction? A. B. OH OH 1.2 moles 2. H3O* C. MgBr هذه معه OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





