
Interpretation:
Numbers are not used to designate the position of the
Concept introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry).IUPAC name consists of three parts, namely Prefix, suffix and root word.
Prefix- Represents the substituent present in the molecule and its position in the root name.
Suffix- Denotes the presence of functional group if any in the molecule. It can be an
Root word - Represents the longest continuous carbon skeleton of the organic molecule.
The functional group in the
The given compound is an aldehyde (
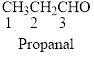
The given compound is an ketone (
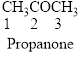
Trending nowThis is a popular solution!

Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
- Write the condensed formulas of the following names of organic compounds. (a). 6-ethyl-4-isopropyl-3-methyloctanearrow_forwardDrawthe characteristic functional group of FOUR of the following six families of organic compounds: Alcohol, Amine, Aldehyde, Ketone, Carboxylic acid, or Ester.arrow_forwardWrite the structural formulas for the reaction of methanol with ethanoic acid. The structural formula for methyl ether is?arrow_forward
- How many hydrogen atoms are in an alkane moleculewith nine carbon atoms? How many are in an alkenewith nine carbon atoms and one double bond?arrow_forwardwhat is the functional group for benzyl alcohol?arrow_forwardWrite structural formulas for all of the possible isomers of n-pentyne that can be formed by moving the position of the triple bond.arrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



