
(a)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.
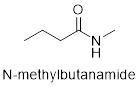
(b)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.

(c)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.

(d)
Interpretation:
The conversion of N-methylbenzylamine to given compound has to be explained.
Concept introduction:
The molecular formula of N-methylbutanamide is
The structure of N- methylbutanamide is as follows.

Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
- QUESTION 1 Which of the following compounds has an activating group? A. Benzoic acid B. None in the answer C. Bromobenzene D. Diphenylamine QUESTION 2 Which of the following compounds has a deactivating group attached to the benzene ring? A. Toluene B. Acetanilide C. Phenol D. Salicyclic Acidarrow_forwardWhich of the following compounds will NOT test positive in a hydroxamic test? a. butanoic acid b. butanoyl chloride c. butyl ethanoate d. butanoic acid anhydridearrow_forwardWhy is methylamine more basic than aniline?arrow_forward
- Reduction of an alkyl azide results in the formation of —-. A. an imine B. an oxime C. a tertiary amine D. a secondary amine E. a primary aminearrow_forward6. The reaction between aniline and nitrous acid at low temperature yields A) an N-nitroso amine B) a diazonium salt C) a nitrile D) an amine nitrite salt 7. An organic nitrogen compound, X, gives ammonia on warming with dilute aqueous sodium hydroxide, X could be A) ethanamide B) ethylamine C) phenylamine D) amino ethanoic acidarrow_forward2. Draw out the following compounds. a. N-methylaniline b. Triisopropylamine c. N,N-dipropylhexylamine d. 1,5-pentanediamine (Also known as Cadaverine for its smell)arrow_forward
- Esters and amides are most easily made by nucleophilic acyl substitution reactions on… A. alcohols B. acid chlorides C. acid anhydrides D. carboxylates E. carboxylic acidsarrow_forwardDescribe the reaction when a. Formaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid. b. Acetaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid. c. Benzaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid.arrow_forward45. Which of the following compounds has the lowest water solubility? a. butanal b. heptanal c. hexanal d. pentanal 48. Which of the following compounds is the most soluble in water? a. butanal b. heptanal c. hexanal d. pentanal 56. Which of the following compounds will undergo oxidation using potassium dichromate to form a carboxylic acid? A a. A and B only b. A and C only c. B only d. C only H B o Carrow_forward
- What are the functional groups present in this antibacterial antibiotic? A. Nitro, phenyl, amine, carbonyl, hydroxyl B. Nitro, phenyl, amine, carbonyl, C. Nitro, phenyl, amine, hydroxyl D. Nitro, phenol, amine, carbonyl, hydroxyl A brief explanation would be highly appreciated + upvotearrow_forwarda. Reaction of acyl compounds with LiAlH4 forms alcohols. b.Reaction of acyl compounds with alcohols forms esters. c.Reaction of carbonyl compounds with LiAlH4 forms alcohols. d.Reaction of carbonyl compounds with alcohols forms hemiacetals. Which are false?arrow_forwardDraw a structure corresponding to each name. a. 2,4-dimethyl-3-hexanamine b. N-methylpentylamine c. N-isopropyl-p-nitroaniline d. N-methylpiperidine e. N,N-dimethylethylamine f. 2-aminocyclohexanone g. N-methylaniline h. m-ethylanilinearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



