
(a)
Interpretation: The products for the given reaction have to be identified.
Concept Introduction:
Ketals:
Ketals are used to protect the carbonyl group of the
In the following example, the carbonyl group of the acetone has been protected as ketal by using ethylene glycol.
Example:
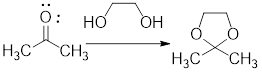
In acidic condition, a ketone reacts with two molecules of alcohol or a molecule of
General scheme:
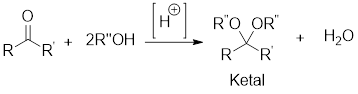
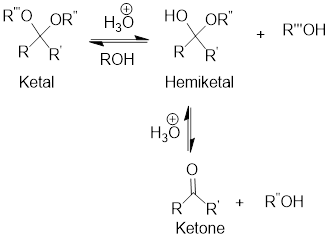
Hydrolysis of Ketal:
Hydrolysis of ketal gives back the corresponding alcohol and the corresponding ketone from which the ketal has been formed. The hydrolysis takes place only in acidic conditions. So, the hydrolysis of ketal is an acid-catalyzed hydrolysis reaction.
General scheme:
(b)
Interpretation: The products for the given reaction have to be found.
Concept Introduction:
Ketals:
Ketals are used to protect the carbonyl group of the ketone.
In the following example, the carbonyl group of the acetone has been protected as ketal by using ethylene glycol.
Example:

In acidic condition, a ketone reacts with two molecules of alcohol or a molecule of diol to form ketal or cyclic ketal respectively.
General scheme:
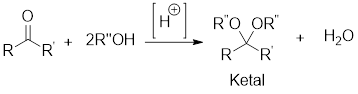
Hydrolysis of Ketal:
Hydrolysis of ketal gives back the corresponding alcohol and the corresponding ketone from which the ketal has been formed. The hydrolysis takes place only in acidic conditions. So, the hydrolysis of ketal is an acid-catalyzed hydrolysis reaction.
General scheme:

Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





