
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 12.8, Problem 22P
(a)
Interpretation Introduction
Interpretation:
The carbonyl compound should be identified for the given amino acid preparation.
Concept introduction:
Amino acid:
An amino acid is an organic molecule and it consists of
The general formula for the amino acid is given below,
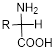
(b)
Interpretation Introduction
Interpretation:
The carbonyl compound should be identified for the given amino acid preparation.
Concept introduction:
Amino acid:
An amino acid is an organic molecule and it consists of amine group
The general formula for the amino acid is given below,
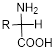
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
arrange the following compound types in order of decreasing ease of hydrolysis: acid halides, acid anhydrides, esters, and amides. Use > in your arrangement.
Using the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.
Write the products of the reaction of diphenhydramine (a base) with the
acid HCI shown below.
H
COCH₂CH₂NCH3 + HC1
CH3
Consider the chemical reaction from the previous question. Are the
reactants or products more soluble in water? Briefly explain.
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Similar questions
- 1. Give the order of basicity of alkaloids based on the R-groups attached to the amino functional group. Based on your knowledge in organic chemistry, explain the reason why one is more or less basic as compared to the other.arrow_forwardDraw the molecules involved in the synthesis of aspirin. Is the forward reaction hydrolysis or condensation? Is the reverse reaction hydrolysis or condensation? Describe what occurs un each type of reaction with respect to acetylsalicylic acid.arrow_forwardWhen hexanoic acid (a carboxlic acid) is heated, a reaction takes place that generates water (which must be removed) and a non-acidic product. What is the product? carboxylate carboxylic acid anhydride ester amide lies! all lies! no reaction will take place!arrow_forward
- Bile salts are derivative of cholestrol. However, the solubilities of these compounds in water are drastically different; cholestrol is highly hydrophobic, and the bile salts are soluble in digestive juices. Explain the differences.arrow_forwardα-Amino acids can be prepared by treating an aldehyde with ammonia/trace acid, followed by hydrogen cyanide, followed by acid-catalyzed hydrolysis. Draw the structures of the two intermediates formed in this reaction.arrow_forwardThe correct IUPAC name of the compound Br Ho CH-C- CH но- -CH=CH2 NH2 OH CH 2-amino-3-bromo-4-formyl-3-hydroxy-5-oxohept-6-enoic acid 0 2-amino-3-bromo-4-formyl-3-hydroxy-5-oxohept-6-eneoic acid 6-amino-5-bromo-4-formyl-5-hydroxy-3-oxohept-1-enoic acid 1-amino-2-bromo-2-hydroxy-4-oxo-5-hexen-3-al-1-oic acid 0 2-amino-3-bromo-4-formyl-3-hydroxy-5-oxo-1-heptene carboxylic acidarrow_forward
- From the given structures which is(a) amide that will release a secondary amine upon hydrolysis? (b) product of hydrolysis of MSO (c) a tertiary amide and (d) a diketonearrow_forwardprovide proper explanation of this question.arrow_forwardGive at least 5 examples of biological compounds having a carboxylic acid functional group and identify the biochemical importance of each compound.arrow_forward
- The structure of Amines are classified as primary (1), secondary (2), and tertiary (3) that concept that seemed difficult to you at first, but then after working on the concept, you were able to master it. Include a description of what made the concept difficult at first, and then discuss what you did in order to better understand the concept.arrow_forwardQ4:- Draw structures, give names, and classify as primary, secondary, or tertiary amine : the eight isomeric amines of formula CaHi.N Q5:- Give structures and names of the principal organic reactants and products of the following reactions. a- conversion of Carboxylic Acids into Esters b- Hydrolysis of nitrites. c- Hofmann degradation of amides Q6:- Arrange the compounds of each set in order of basicity ? Explain your choice. ethylamine, 2-aminoethanol, 3-amino-l-propanolarrow_forwardFeatures of the structure of imino acids. Formation of amides from amino acids.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning