
(a)
Interpretation:
It should be determined that the amide used to produce Benzylmethylamine on reaction with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an
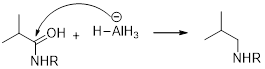
(b)
Interpretation:
It should be determined that the amide used to produce Ethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
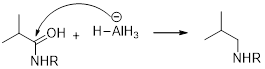
(c)
Interpretation:
It should be determined that the amide used to produce Diethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
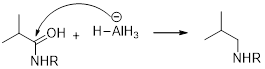
(d)
Interpretation:
It should be determined that the amide used to produce Triethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
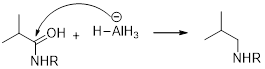
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
- Draw condensed and skeletal structures for each of the following amines a. 2-methyl-N-propyl-1-propanamine b. N-ethylethanamine c. 5-methyl-1-hexanamine d. methyldipropylaminee. e. N,N-dimethyl-3-pentanamine f. cyclohexylethylmethylaminearrow_forwardWhat amides would you react with LiAlH4 to form the following amines? a. benzylmethylamine c. diethylamine b. ethylamine d. triethylaminearrow_forwardExplain aryl and alkyl amines and their reactions by giving two examples of each.arrow_forward
- A. 2,4-dihydroxybenzaldehyde reacts with ethylamine to form an imine. B. 2,4-dihydroxybenzaldehyde reacts with ethanamide to form an ester. Are the statements True?arrow_forwardThe hydrolysis of an amide in acidic conditions forms A. a carboxylate salt and an alcohol B. a carboxylate salt and an amine C. an alcohol and an amine salt (an ammonium ion) D. a carboxylic acid and an amine salt (an ammonium ion)arrow_forwardReduction of ethyl nitrile using H2/Ni produces a.1-propanamine b.propanamine c.ethanamine d.butanamine Reduction of nitrobenzene and its further reaction with NaOH producesa.1-propanamine b.aniline c. ethyl amine d. n-butyl aminearrow_forward
- Amide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forwardPredict the chemical name of compound B a. p-chlorobenzylamine b. 4-bromoaniline c. benzylamine d. p-chloronitrile e. p-chlorobenzaldehyde 2. What is the reaction name for the chemical transformation of A to B a. reductive amination b. catalytic reduction c. carbonyl dehydration d. Hofmann elimination e. Aldehyde rearrangementarrow_forwardWhat type of amine is N-methylmorpholine? O A. Primary O B. Secondary OC. Tertiary OD. Quaternary O E. None of these choices.arrow_forward
- N-p-hydroxyphenylethanamide is commonly known as a. acetaminophen b. acetamide c. acetanilide d. formamide High molar mass amines have __________ odor. a.strong ammoniacal b.fruity c.fishy d.obnoxious Trimethyl amine has _________ odor. a.obnoxious b.fishy c. ammoniacal d. fruityarrow_forwardReduction of primary amide will form _____ amine. a. Primary b. Secondary c. Tertiary d. mides cannot undergo reductionarrow_forwardDescribe the water solubility of amines in relation to theircarbon chain length.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


