
Concept explainers
(a)
Interpretation:Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
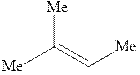
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
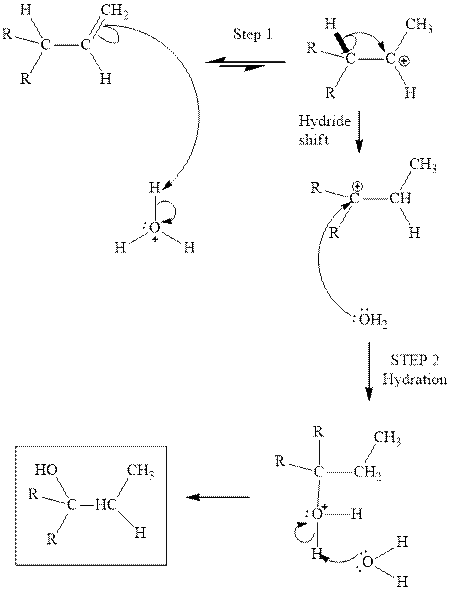
(b)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
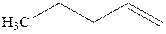
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
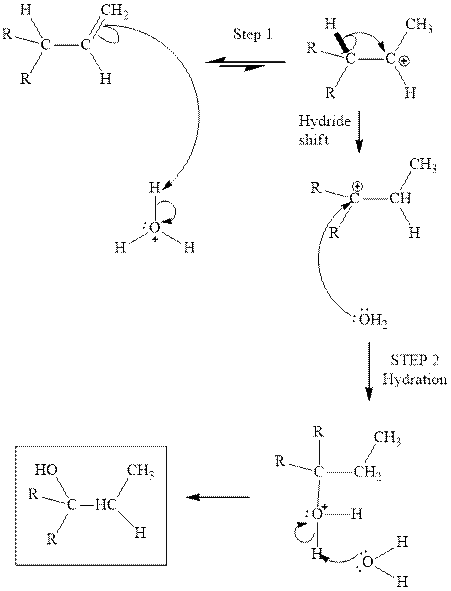
(c)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
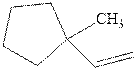
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
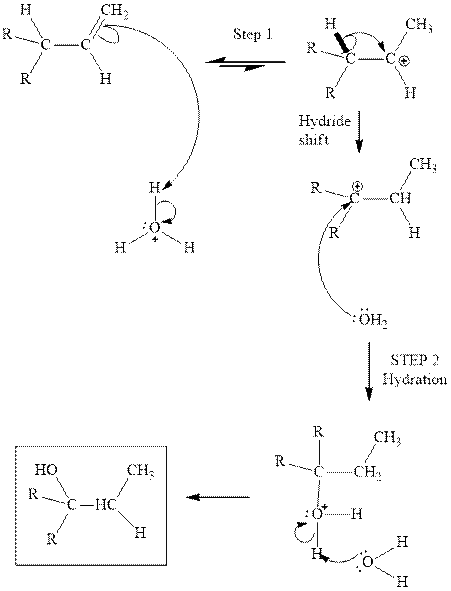
in the first stage. In the second stage, water itself acts as a nucleophile and another water abstracts a proton to give final hydration product as illustrated below.
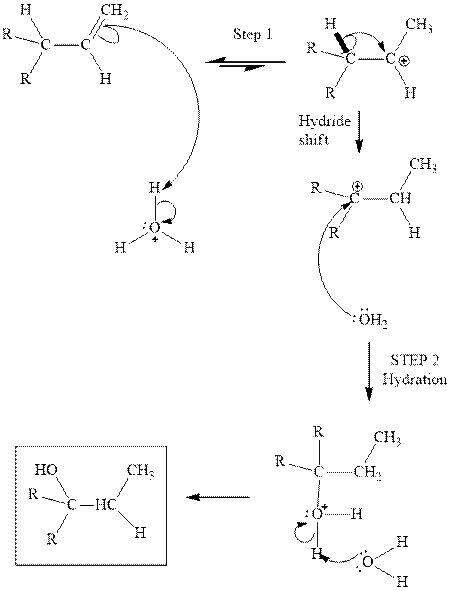
(d)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
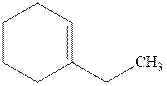
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
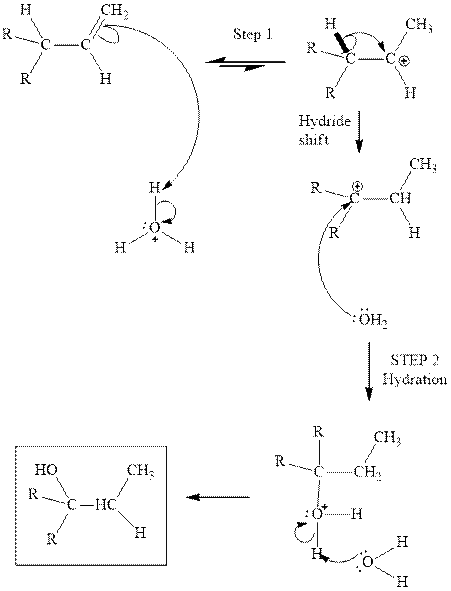
(e)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Identify the characteristics of the acid-catalyzed hydration of an alkenearrow_forwardProvide the major organic product of the following reaction. H3O', heatarrow_forwardprovide the synthesis of Oleic acid from any straight chain alkanes containing 5 carbons or less. must make all reactants containing carbon from a straight chain alkane containing 5 carbons or less, but may utilize any reagents or solvents needed without making them.arrow_forward
- Provide the principle organic product for the following reactions. If more than one product is formed indicate which one you expect to be the major product.arrow_forwardWhat two alkenes give rise to each alcohol as the major product of acid-catalyzed hydration?arrow_forwardIndicate the products obtained when reactinga) Ethyl ethanoate b) Ethyl benzoatewith 1 or 2 (in each case) equivalents of CH3MgI/Et2Oarrow_forward
- Provide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the following reactions.arrow_forwardExplain what is meant by reduction in organic reactionsand provide an example of a reduction reaction involvingan aldehyde or ketone.arrow_forwardState and useMarkovnikov's rule and a displayed/structural formula tosuggest the dominant product when hydrogen chloride reacts with 2-methylbut-2-ene. Provide the IUPAC name of the product obtainedarrow_forward
- Write the structure of the principal organic product of each reactionarrow_forwardThe conversion of alcohols into alkyl halides by reaction with hydrogen halides is an example of a Nucleophilic Substitution Reaction. This kind of reaction can proceed by two different mechanisms depending on the structure of alcohol substrates used. Generally, primary alcohols are substituted via SN2 mechanism, while secondary and tertiary alcohols undergo SN1 mechanism. Consider the following reaction given in the picture below and the questions in the picture too.arrow_forwardGive the major organic product(s) for each of the following reactions. Write NR if a reaction will not occur.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
