
(a)
Interpretation:Whether norbornene is chiral or not should be explained.
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system ispresence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
Four kinds of symmetry elements that may be present are tabulated as follows:
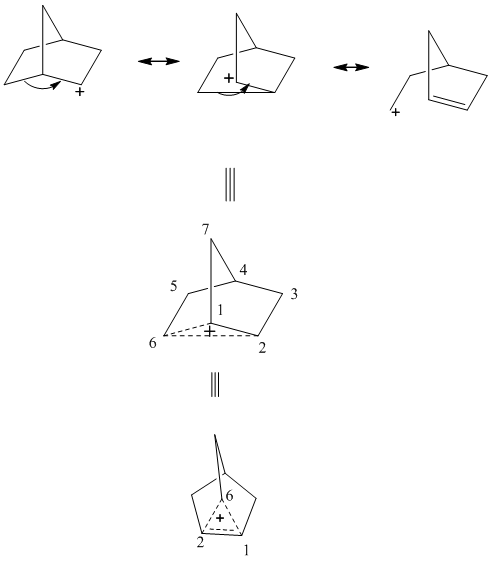
The non-classical cation is unique carbocation that involves delocalization of electron density of filled bonding orbital over three carbons. It exists for systems such as phenonium ions,norbornyltosylate, brosylate, and nortricyclonium cations. They can be understood as carbocation that has transition state for two asymmetric equilibrium cation derived from anchimeric-assistance that represents neighboring group participation in the stabilization of positive charge as illustrated below.
(b)
Interpretation:Three chiral centers in
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system ispresence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
(c)
Interpretation: Chemical structure for the stereoisomer of
Concept introduction: Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system isthe presence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- 7. Dihydrocarveol is another natural product found in spearmint oil, but in minor amounts. dihydrocarveol a) Determine the R or S configuration of each chiral center in dihydrocarveol. b) When separating the components of spearmint oil by column chromatography, in what order would you expect carvone, limonene, and dihydrocarveol to elute? c) Which compound would you expect to have the lowest Rf value by TLC: carvone, limonene, or dihydrocarveol?arrow_forwardConsider the stereochemistry of the two compounds and its relation to optical activity. (25%) (75%) Which statement is true? The mixture is optically active. The mixture is optically inactive. There is not enough information to determine whether the mixture is optically active or not. Ill ...||arrow_forward1. If life arose by “chemical evolution” (which is supposed to be new chemical compounds arising by random chance), what should be the proportion of each of the L- and D-enantiomers present in chiral compounds produced by random chance? How does this match with what we see in living systems? What conclusion can you make from this about the likelihood of life arising by “chemical evolution?" 2. Why does chirality make a difference when new medications are developed? What is an ethical way of addressing this concern in the development process? What is the connection with the way the body is designed?arrow_forward
- The active enantiomer of aryloxypropanolamines is the S-form, whereas the activeenantiomer of arylethanolamines is the R-form. Does this imply that the twoagents are binding differently to the binding site?arrow_forwardExplain why the enantiomers of 1,2-dimethylaziridine can be separated even though one of the “groups” attached to nitrogen is a lone pair.arrow_forward26. Draw the structure of the product(s) of the following stereospecific reaction. Assign the configuration (R or S) of any chiral centers (use the text command on Marvin Sketch) and indicate whether or not the product mixture is optically active and why.arrow_forward
- a. What is the classification of the Arabinose in terms of combined no. of carbons and highest functional group present? b. Provide the Cahn-Ingold-Prelog (R.S) Configuration of all the Chiral C present in the structure given above. c. State a Function of arabinose.arrow_forward4 What are enantiomers? What instrument can you use to distinguish the enantiomers? What is racemic mixture? What is enantiomeric excess (e.e)? 5 For a chiral compound with 2 chiral centers how many configurations (also called isomers in lose sense) are possible. Use 2¹ rule. 6 Pure (R)- lactic acid has rotation of + 12.31º. A mixture of lactic acid has a specific rotation of +2.71º. Determine the percent composition of two enantiomers (R) and (S) in the mixture.arrow_forward10- Which of the following compounds would give positive Tollens' test? A. (CH3)3 COH B. H₂CO C. (CH3)2CO D. CH3COOH 11- Choose the correct statement: to y Dut (ii) (iii) (iv) (1) A. (iii) and (iv) are chain isomers B. (i) and (iv) are functional isomers C. (ii) and (iv) are chain isomers D. (i) and (ii) are functional isomers 12- Choose the common name for the following molecule: 0 A. 3-Methylbutanone B. 2-Methylbutanone C. Methyl propyl ketone D. Isopropyl methyl ketone 13-(CH3)2CHCHO is the final product resulting from oxidation of X in presence of Y. What are X and Y? A. X: Isobutyl alcohol and Y: CrO3/H2SO4(aq) B. X: sec-Butyl alcohol and Y: PCC/CH₂Cl2 C. X: Isobutyl alcohol and Y: PCC/CH₂Cl2 D. X: sec-Butyl alcohol and Y: CrO3/H2SO4(aq)arrow_forward
- 19. A sample of the chiral molecule limonene is 82% enantiopure. What is the percentage of major and minor enantiomers present in the mixture? What is the percent enantiomeric excess?arrow_forwardA mixture contains equal amounts of compounds A–D. a.Which compounds alone are optically active? b. If the mixture was subjected to fractional distillation, how many fractions would be obtained? c.How many of these fractions would be optically active?arrow_forwardConsider the following experiment. An a,B-unsaturated ketone (X) was prepared by refluxing 1.60 g of 2-acetyl-5-chlorothiophene (mp 46-49 oC, molar mass 160 g/mol), 1.79 g of 4- (dimethylamino)benzaldehyde (mp 72-75 oC, molar mass 149 g/mol) in a solution of 25 mL methanol and 0.8 g of sodium hydroxide. During the reflux, the reaction mixture was monitored for completion at 15 min intervals. After 90 min. the reaction was found to be about 95 % complete with a small amount of one of the reactants remaining unreacted in the reaction mixture. *After 90 minutes the mixture was allowed to cool and then acidified with 23 mL of 1 M HCl solution, and the resultant acidified mixture was rotary evaporated. The resultant mixture was transferred to a 125 mL separatory funnel and was extracted twice with 25 mL of chloroform. The combined chloroform fractions was washed twice with 20 mL water, then dried with a chemical drying agent and finally filtered. The filtrate was transferred to a round…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





