
Concept explainers
a.
Interpretation: The hybridization state and geometry for each carbon atom in the following compound should be identified:
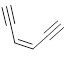
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form
b.
Interpretation: The hybridization state and geometry for each carbon atom in the following compound should be identified:
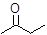
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Identify the following alkyl groups: a. b. CH3CH2CH2CH2 c. d. CH3arrow_forwardWrite a condensed structural formula for the following compounds: a. b.arrow_forwardLipoic acid is required by many microorganisms for proper growth. As a disulfide, it functions in the living system by catalyzing certain oxidation reactions and is reduced in the process. Write the structure of the reduction product.arrow_forward
- 1.Discuss how aromaticity is affected in large ring compounds. 2. Discuss the aromaticity of heterocyclic compounds. 3. Discuss the aromaticity of polynuclear hydrocarbonsarrow_forwardGive the IUPAC name of the following compounds: a. SH b. HSarrow_forwardProvide the correct IUPAC name for each compound below. b.arrow_forward
- The most common factor at the structural level that lowers the boiling point among compounds that are isomers is: a.)valence electrons b.)hybridization c.)hydrogen bridges d.)ramifications And why ?arrow_forwardFumaric acid is produced by alcohol A or aldehyde B, while maleic acid is produced by alcohol C or aldehyde D. Draw the skeletal structure of A,B,C and Darrow_forward1. Give the IUPAC name for the following structures: a. b. C d. Karrow_forward
- 12) What is the major difference between an antiaromatic and aromatic compound? A) The structure must be cyclic for aromatic but not antiaromatic compounds? B) Antiaromatic compounds have at least one sp3 hybridized atom in the ring C) Antiaromatic compounds can assume a chair-like structure while aromatic compounds are nearly flat D) Aromatic compounds cannot have a charged atom in the structure E) Only aromatic compounds follow Huckle's rule.arrow_forward1. Write the correct IUPAC names for the following organic compounds: a) b)arrow_forwardF. Br The IUPAC name is:arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





