
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Question
Chapter 1.10, Problem 8LTS
Interpretation Introduction
Interpretation: The hybridization state of each carbon in the following compound needs to be determined:
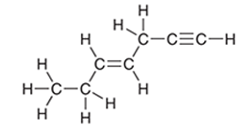
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
what is the hybridization of each carbon atom in toluene and how many S orbitals are present within the compound?
Identify the hybridization state, molecular geometry and approximate bond angle at the indicatednitrogen atom in the following
compound.
O sp²,bent, 109°
O sp²,bent, 120°
O sp3, tetrahedral, 109.5°
O sp3, trigonal pyramidal, ~109.5°
O sp2, trigonal pyramidal, 120°
CH3 HH CH₂.
H₂=C₂C=C₂C₂O₂H
N
OU!
H³N¬C-C¬-H
H
HH
.0.
What is the hybridization of each carbon atom in the following structure.
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- There are two compounds with the molecular formula HN3. One is called hydrogen azide; the other is cyclotriazene. (a) Write the Lewis structure for each compound. (b) Designate the hybridization of each nitrogen in hydrogen azide. (c) What is the hybridization of each nitrogen in cyclotriazene? (d) How many sigma bonds are in hydrogen azide? In cyclotriazene? (e) How many pi bonds are in hydrogen azide? In cyclotriazene? (f) Give approximate values for the N-to-N-to-N bond angles in each molecule.arrow_forwardDraw three contributing structures of the following compound (called guanidine) and state the hybridization of the four highlighted atoms. In which orbitals do the three lone pairs drawn reside?arrow_forwardExplain why a carbon atom cannot form five bonds using sp3d hybrid orbitals.arrow_forward
- AmbienTM is a sedative used in the treatment of insomnia. It was discovered in 1982 and brought to market in 1992 (it takes a long time for new drugs to undergo the extensive testing required to receive approval from the Food and Drug Administration). Identify the hybridization state and geometry of each carbon atom in the structure of this compound:Write the carbon atom(s) in numerical order as a comma separated list (e.g. to identify carbon atoms 1, 3, 4, 5, 18 and 19 write "1,3-5,18-19"). If there are no carbons with the specified hybridization, write "0" for carbon atom(s) and select "N/A" for the geometry.arrow_forwardDraw the orbital overlap diagram for H H-C-N=C=O H And identify the hybridization for each of the carbon, nitrogen and oxygen atomsarrow_forwardPredict the hybridization and the bond angles around the indicated atoms. H b NEC-0-Ċ-H methyl cyanate Atom a Atom b Atom c Hybridization Bond anglearrow_forward
- Draw the molecular orbital picture of CH3 - C= C - H. Indicate the type of hybridization on each carbon atom.arrow_forwardIdentify the hybridization state, molecular geometry and approximate bond angle around the carbon atomfor the molecule shown in box below. sp², tetrahedral, 109° sp², trigonal planar, 120° sp³, tetrahedral, 109.5° sp³, trigonal pyramidal, <109.5° O sp², trigonal pyramidal, 180° HCOOHarrow_forwardIdentify hybridization in each Carbon atom of given molecules.arrow_forward
- A student investigates the physical and chemical properties of various carbon-containing compounds. The complete Lewis electron-dot diagrams and boiling points for two compounds, Q and Z, are shown in the following table: a) Identity the hybridization of the valence orbitals of the carbon atom in compound Q that is indicated by the arrow in the diagram.arrow_forwardProvide the hybridization, electronic geometry (shape, ex. Tetrahedral), and bond angle for each of the indicated atoms in the molecule below. C2 C1 H-C-CEN : Atom Hybridization Shape Bond Angle C1 C2arrow_forwardIdentify all of the carbon atoms that are sp2-hybridized in the following molecule:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY