
Interpretation: The number of carbon atoms and
-bonds present in the following compound should be identified:
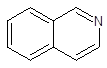
Concept Introduction: A method used to represent molecular structures of compounds is said to be bond line notation. In this notation, a line depicts a bond between two atoms and are drawn in a zigzag format. Atoms other than carbon and hydrogens are specifically depicted in this notation. It is assumed that carbon atoms are bonded to enough hydrogen atoms that are required to complete the octet.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- 3-25 Why are carbon and silicon reluctant to form ionic bonds?arrow_forwardAre the bonds in each of the following substances ionic,nonpolar covalent, or polar covalent? Arrange the substanceswith polar covalent bonds in order of increasing bond polarity:(a) KCl (b) P₄(c) BF₃(d) SO₂(e) Br₂(f) NOarrow_forwardWhich bond in each of the following pairs of bonds is the strongest?(a) C–C or C = C(b) C–N or C ≡ N(c) C ≡ O or C = O(d) H–F or H–Cl(e) C–H or O–H(f) C–N or C–Oarrow_forward
- Draw a Lewis structure for each of the following molecules and ions. In each case, the atoms can be connected in only one way. (a) Br2 (b) H2S (c) N2H4 (d) N2H2 (e) CN- (f) NH4+ (g) N2 (h) O2arrow_forwardWhich two species have the same number of lone electron pairs in their Lewis structures?(a) H2O and H3O+(b) NH3 and H3O+(c) NH3 and CH4(d) NH3 and NH4+arrow_forwardDraw a Lewis Structure for each of the following species and assign formal charge where appropriate. Using electronegative values from the period table that was provided identify polar covalent bonds and label the atoms δ+ and δ−. For each of the molecules indicate whether or not it has a dipole moment. (a)CH5N (b) HCN (c) H2CO (d) CH3NC(e) CH3SOCH3 (f) H6BNarrow_forward
- Oxalic acid, H2C2O4, a poisonous colorless solid, is found in some vegetables such as spinach and rhubarb. It is present in concentrations well below the toxic limit, so you can't use this as a reason to refuse a helping of spinach. The order of atoms in a molecule of oxalic acid is HO2CCO2H. (a) How many unshared pairs of electrons are on each of the carbon atoms? (b) How many unshared pairs of electrons are on each of the oxygen atoms?arrow_forwardFrom their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:(a) As, H, N, P, Sb(b) Cl, H, P, S, Si(c) Br, Cl, Ge, H, Sr(d) Ca, H, K, N, Si(e) Cl, Cs, Ge, H, Srarrow_forwardArrange each set of bonds in order of increasing polarity, and indicate bond polarity with δ+and δ- symbols: (a) Cl-F, Br-Cl, Cl-Cl (b) P-F , Si-F, S-F and Arrange each set of bonds in order of decreasing polarity, and indicate bond polarity with a polar arrow: (a) Se-Cl, Se-F, Se-Br (b) S-B, F-B, Cl-Barrow_forward
- Judging from their relative positions in the Periodic Table, which atom in each set is more electronegative? (a) Carbon or nitrogen (b) Chlorine or bromine (c) Oxygen or sulfurarrow_forwardUsing the bond energies as shown, determine the approximate enthalpy change for each of the following reactions:(a) Cl2(g) + 3F2(g) ⟶ 2ClF3(g)(b) H2 C = CH2(g) + H2(g) ⟶ H3 CCH3(g)(c) 2C2 H6(g) + 7O2(g) ⟶ 4CO2(g) + 6H2 O(g)arrow_forwardDraw Lewis structures for the following compounds. Remember to enclose ions in square brackets. On your answer sheet, state the number of valence electrons in each compound. (a) NaCN (b) CH 3 Br (c) Ca(OCl) 2arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
