
a.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
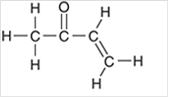
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form
b.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
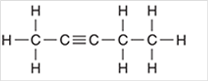
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
c.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
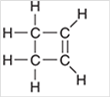
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
d.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
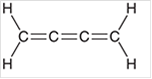
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
e.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
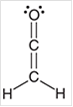
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- A. The overlap of atomic orbitals leads to the formation of bonds. Explain what kind and how many orbitals are used to build the hybrid orbitals of a carbon atom in ethylene to give rise to the three sigma bonds. Also, what happens to the orbital(s) not used in the hybridization process? B. What is the relative energy of the hybrid orbitals used by carbon of ethylene in bonding with respect to the original atomic orbitals of carbon in a ground state?arrow_forwardPlease help to identify the hybridization of each carbon in the structurearrow_forwardWrite a bond-line structure for the following compounds. Present correct geometry for sp3, sp2, andsp hybridized carbon atoms. b.-OOCCHCHCCCH2COOH, The molecule is negatively charged, the double bond has transgeometryarrow_forward
- What are the hybridizations of the Carbons indicated by the arrows?arrow_forwardGive the shape that describes each hybrid orbital set.arrow_forwardCH3+ and CH3- are two highly reactive carbon species. a. What is the predicted hybridization and geometry around each carbon atom? b. Two electrostatic potential plots are drawn for these species. Which ion corresponds to which diagram and why?arrow_forward
- Identify the hybrid orbital on each C and O. Indicate the geometry and bond anglearound each of these atomsarrow_forwardDraw the energy diagram of the hybridized atomic orbitals of the oxygen atoms for a. CH3OCH2CH3 (ethyl methyl ether) b. CH3COCH3 (acetone)arrow_forwardIndicate the hybridization of each of the carbon atoms in the structure below.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
