
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
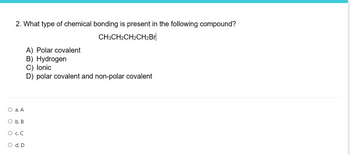
Transcribed Image Text:2. What type of chemical bonding is present in the following compound?
CH3CH2CH2CH2Br
O a. A
O b. B
O C. C
O d. D
A) Polar covalent
B) Hydrogen
C) lonic
D) polar covalent and non-polar covalent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7.97 Consider the structure shown below for as well as any other important resonance structures. (a) What is the expected O—N—O bond angle in this structure? (b) The molecule contains N—O bonds of two different lengths. How many sborter N—O bonds would be present?arrow_forwardWhat is meant by a chemical bond? Why do atoms form bonds with each other? Why do some elements exist as molecules in nature instead of as free atoms?arrow_forwardWhich of the following statements is false concerning bonding? Elements with extremely different electronegativities tend to form ionic bonds with each other. In an N—O bond. electron density is greater near the O atom. An N—O bond is an example of a polar covalent band. In general, chemical bonds form to minimize energy. The bond in KBr is formed by sharing electrons.arrow_forward
- Write Lewis structures for the following: (a) ClF3 (b) PCl5 (c) BF3 (d) PF6arrow_forwardWhy is the geometric structure of a molecule important, especially for biological molecules?arrow_forwardCarbon monoxide (CO) forms bonds to a variety of metals and metal ions. liS ability to bond to iron in hemoglobin is the reason that CO is so toxic. The bond carbon monoxide forms to metals is through the carbon atom: MCO a. On the basis of electronegativities, would you expect the carbon atom or the oxgen atom to form bonds to metals? b. Assign formal charges to the atoms in CO. Which atom would you expect to bond to a metal on this basis? c. In the MO model, bonding MOs place more electron density near the more electronegative atom. (See the HF molecule in Figs. 4-54 and 4-55.) Antibonding MOs place more electron density near the less electronegative atom in the diatomic molecule. Use the MO model to predict which atom of carbon monoxide should form bonds to metals.arrow_forward
- Write Lewis structures for: (a) PO43 (b) ICl4 (c) SO32 (d) HONOarrow_forwardThe molecular ion S3N3 has the cyclic structure All SN bonds are equivalent. (a) Give six equivalent resonance hybrid Lewis diagrams for this molecular ion. (b) Compute the formal charges on all atoms in the molecular ion in each of the six Lewis diagrams. (c) Determine the charge on each atom in the polyatomic ion, assuming that the true distribution of electrons is the average of the six Lewis diagrams arrived at in parts (a) and (b). (d) An advanced calculation suggests that the actual charge resident on each N atom is 0.375 and on each S atom is +0.041 . Show that this result is consistent with the overall +1 charge on the molecular ion.arrow_forwardG. N. Lewis developed a model for chemical bonding that you have learned in this chapter. His theory was extremely successful and is used today at all levels of chemistry, from the introductory class to the research laboratory. Why was Lewis theory so successful?arrow_forward
- Which one of the following bonds is polar? a. a bond between two identical atoms b. a bond between two atoms with the same electronegativity c. a bond between two atoms with different electronegativitiesarrow_forward6. CO2 a. Lewis structure e. aloq hud c. Electronic geometry_ d. Molecular shape_ Bond angle? b. Draw the different bonds and label their polarity.arrow_forward8. SO3 a. Lewis structure c. Electronic geometry_ d. Molecular shape. e. Bond angle? b. Draw the different bonds and label their polarity. Lyzemoag bihomosi narkishpalom.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning