
(a)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(a)
Answer to Problem 129A
The electron dot structure of 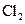 is given as follows:
is given as follows:
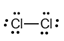
Explanation of Solution
The bonding
The given substance is 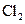
The electron dot structure of 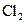 is given as follows:
is given as follows:
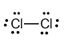
There is a single bond between 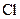 and
and 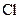
The valency of 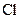 is seven.
is seven.
Since one electron is involved in bonding, the remaining six valence electrons form three lone pairs.
(b)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(b)
Answer to Problem 129A
The electron dot structure of 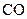 is given as follows:
is given as follows:
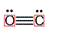
Explanation of Solution
The bonding atoms, the number of bonds in the molecule, and the lone pairs still present in the bonding atoms are all described by an electron dot structure.
The given substance is 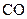
The electron dot structure of 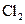 is given as follows:
is given as follows:
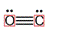
There is a triple bond between 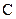 and
and 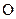
Since three electrons in C is involved in bonding, the remaining valence electrons form one lone pair.
(c)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(c)
Answer to Problem 129A
The electron dot structure of 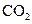 is given as follows:
is given as follows:

Explanation of Solution
The bonding atoms, the number of bonds in the molecule, and the lone pairs still present in the bonding atoms are all described by an electron dot structure.
The given substance is 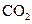
The electron dot structure of 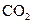 is given as follows:
is given as follows:

There is a double bond between 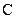 and
and 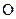
The valency of 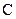 is four
is four
Since four electron is involved in bonding, there is no lone pairs of electrons in carbon.
There are two lone pairs of electrons in oxygen.
(d)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(d)
Answer to Problem 129A
The electron dot structure of 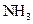 is given as follows:
is given as follows:
Explanation of Solution
The bonding atoms, the number of bonds in the molecule, and the lone pairs still present in the bonding atoms are all described by an electron dot structure.
The given substance is 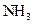
The electron dot structure of 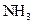 is given as follows:
is given as follows:
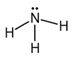
There is a single bond between 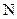 and
and 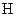
The valency of 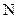 is five.
is five.
Since three electrons of nitrogen is involved in bonding, the remaining two valence electrons form one lone pair.
(e)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(e)
Answer to Problem 129A
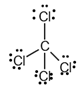
The electron dot structure of 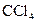 is given as follows:
is given as follows:
Explanation of Solution
The bonding atoms, the number of bonds in the molecule, and the lone pairs still present in the bonding atoms are all described by an electron dot structure.
The given substance is 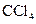
The electron dot structure of 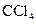 is given as follows:
is given as follows:
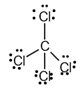
There is a single bond between 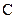 and
and 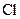
The valency of 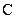 is four.
is four.
Since four electrons are involved in bonding, there is no lone pair in carbon.
(f)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(f)
Answer to Problem 129A
The electron dot structure of 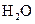 is given as follows:
is given as follows:
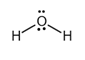
Explanation of Solution
The bonding atoms, the number of bonds in the molecule, and the lone pairs still present in the bonding atoms are all described by an electron dot structure.
The given substance is 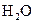
The electron dot structure of 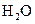 is given as follows:
is given as follows:
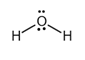
There is a single bond between 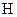 and
and 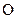
The valency of 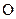 is six
is six
Since two electron is involved in bonding, the remaining four valence electrons form two lone pairs.
(g)
Interpretation: The electron dot structure for the substance is to be drawn.
Concept Introduction: If the compound's molecular formula is known, electron dot structures or the Lewis dot formulas can be drawn. The type of bond and the location of the atoms in a molecule that are related to one another are determined by the electron dot structure.
(g)
Answer to Problem 129A
The electron dot structure of 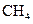 is given as follows:
is given as follows:
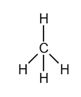
Explanation of Solution
The bonding atoms, the number of bonds in the molecule, and the lone pairs still present in the bonding atoms are all described by an electron dot structure.
The given substance is 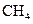
The electron dot structure of 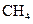 is given as follows:
is given as follows:
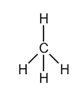
There is a single bond between 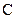 and
and 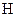
The valency of 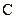 is four.
is four.
Since four electrons are involved in bonding, there is no lone pairs of electron in carbon.
Chapter 9 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





