
(a)
Interpretation:
The Haworth projections for
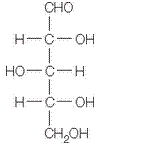
in a-furanose form should be identified along with the name of the sugar.
Concept introduction:
A Haworth projection is common way to represent the cyclic structure of monosaccharides.
Five and six membered hemiacetals are represented as planar pentagons or hexagons.
In the Haworth projection
In the Haworth projection
For example:
Haworth projections of glucose:
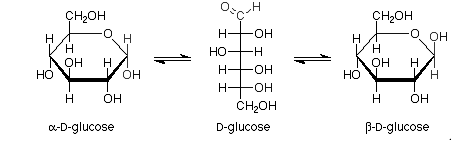
Pictorial representation:
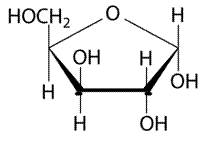
(a)
Explanation of Solution
The given structure is as follows.
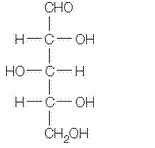
Above structure has 5 carbons and on 3rd carbon
Let’s draw the Haworth projection of
In the D form of Haworth projection, the exocyclic O group at the anomeric centre is on the opposite face as to the
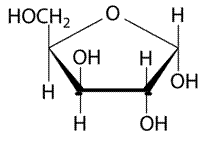
(b)
Interpretation:
The Haworth projections for L isomer of (a) should be drawn.
Concept introduction:
A Haworth projection is common way to represent the cyclic structure of monosaccharides.
Five and six membered hemiacetals are represented as planar pentagons or hexagons.
In the Haworth projection
In the Haworth projection
For example:
Haworth projections of glucose:
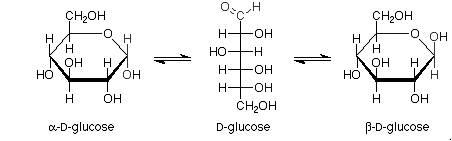
Pictorial representation:
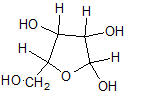
(b)
Explanation of Solution
In L form of Haworth projection, the exocyclic O group at the anomeric centre is on the same face as to the
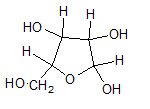
(c)
Interpretation:
The Haworth projections for a-D-Glc-NAc should be drawn.
Concept introduction:
A Haworth projection is common way to represent the cyclic structure of monosaccharides.
Five and six membered hemiacetals are represented as planar pentagons or hexagons.
In the Haworth projection
In the Haworth projection
For example:
Haworth projections of glucose:
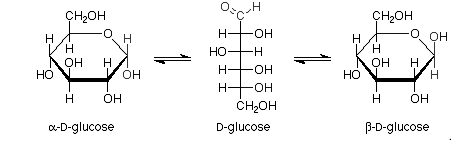
Pictorial representation:
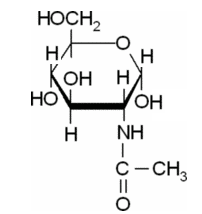
(c)
Explanation of Solution
In this compound,
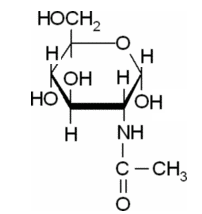
(d)
Interpretation:
The Haworth projections for a-D-Fructofuranose should be drawn.
Concept introduction:
A Haworth projection is common way to represent the cyclic structure of monosaccharides.
Five and six membered hemiacetals are represented as planar pentagons or hexagons.
In the Haworth projection
In the Haworth projection
For example:
Haworth projections of glucose:
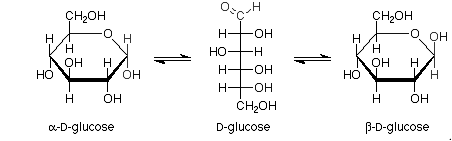
Pictorial representation:
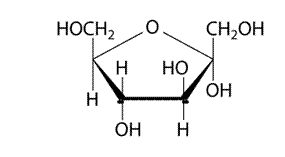
(d)
Explanation of Solution
It has 5 member furanose ring. The structure is as follows.
Want to see more full solutions like this?
Chapter 9 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





