
Interpretation:
Formula for ionic compound formed by cesium and nitride needs to be determined.
Concept introduction:
Ionic compounds are formed as the result of formation of positive as well as negative ions. Electrons are basically transformed from one atom to other for formation of rare gas electron structure for each ion.
The atom that forms a positive ion will loses electrons to the atom that basically gains electrons for formation of a negative ion.
Answer to Problem 22PP
Formula is
Explanation of Solution
Formula for the ionic compounds formed by cesium and nitride is
The charge on Cs ion is +1 and that on N ion is -3 thus, to from neutral compound, 1 mol of N ion should react with 3 mol of Cs ion.
Chemical equation is represented as follows:
Here, 3 electrons from 3 Cs (1 electron each) are transferred to 1 N atom.
The electronic configuration is represented as follows:
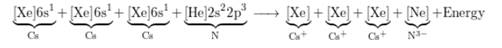
Therefore,
Chapter 7 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
The Cosmic Perspective (8th Edition)
Microbiology: An Introduction
Chemistry: Structure and Properties (2nd Edition)
Microbiology: An Introduction
Campbell Biology (11th Edition)
- 1. Ampicillin is an antibiotic used to prevent and treat several bacterial infections. Bacampicillin is a derivative of ampicillin (a prodrug) that enhances the oral bioavailability of the parent antibiotics. In the body, Bacampicillin decomposes to release Ampicillin and side products. Provide a mechanism for the following reaction. For simplicity, replace the core structure with R. NH2 Bacampicillin N NH2 S H3O+ Ampicillin OH Η CO2 Ηarrow_forwardF Draw the starting structure that would yield this product under these conditions. Drawing 1. NH4Cl, NaCN 2. HCI, H2O, heat NH3 + L 00 O Problem 29 Atoms, Bonds and Rings Draw or tap a new boarrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- K Draw the major product of this reaction. Ignore inorganic byproducts. 1. HNO3, H2SO4 2. SnCl2, ethyl acetate Drawing aarrow_forward6c3. Please solve the following problem, give the relevant equation(s) used and explanation.arrow_forwardDetermine the value of K, at 25 °C for the reaction 2H₂O + 2Cl₂ = 4H+4C1+02 1.36 HOarrow_forward
- Please correct answer and don't use hand ratingarrow_forwardShow the product of Step 2, including lone pairs. OH PCC 1. CH,MgBr Product 1 Product 2 2. H₂O+ Step 1 Step 2arrow_forward2. Bamboo shoots contain cyanogenic glycosides named taxiphyllin. Taxiphyllin is considered a poison because it liberates toxic cyanide (CN) upon hydrolysis. a. Draw arrow-pushing mechanism for this hydrolysis reaction and the production of cyanide. Use HA as needed. HO OH .OH HO OH H CN b. What is the driving force for this reaction?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





