
Interpretation:
The formation of ionic compound from elements of group 1 and group 15.
Concept introduction:
Chemical compounds are formed when the atoms, ions or molecules of two substances are attracted towards each other forming a
Answer to Problem 11PP
The ionic compound formed is of type 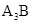
Explanation of Solution
The periodic table depicts the position of the group 1 and group 15 elements.
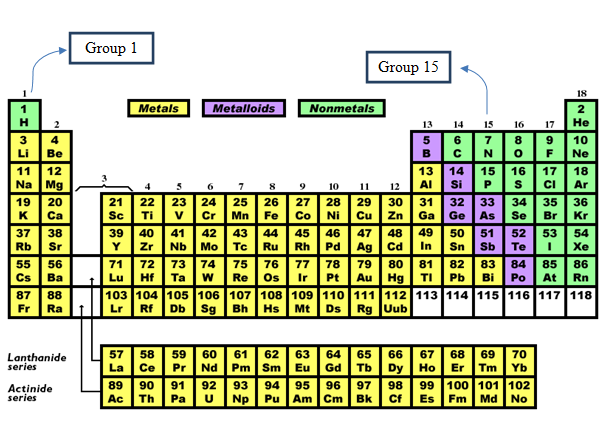
Figure 1
As per overall periodic trends, the atomic and ionic radii increase on going down the group.
The group 1 elements are also said to be alkali metals. It has electronic configuration of. It has one valence electron in its valence shell. These have tendency to form ionic compounds due to its low ionization energy. The outmost electron is lost easily and hence tends to form +1 charged particles or monovalent ions.
The group 15 elements are also said to be pnicogens. It has electronic configuration of. It has 5 valence electrons in outermost shells. It can accept electrons and become negatively charged particles.
There is transfer of electron or gain of electrons, causing the atoms to become positively charged or negatively charged. These opposing charged particles will attract one another to form ionic compound. The compounds between group 1 and group 15 are formed when there is exchange of ions that results in formation of ionic compounds
Here group 1 element\ will lose one electron to become an ion with +1 charge. Group 15 element will gain 3 electrons to become ion with -3 ion. These oppositely charge ions will attract each other to form a compound as 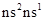 where A represents group 1 atom and B represents group 15 atom.
where A represents group 1 atom and B represents group 15 atom.
The ionic compound formed is of type 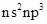
Chapter 7 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Microbiology: An Introduction
Organic Chemistry (8th Edition)
Anatomy & Physiology (6th Edition)
Campbell Biology (11th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
- 120 100 20 20 bound drug/free drug (%) 60 40 60 80 80 0 0 Scatchard Plot of Drug Binding 20 20 40 60 80 100 120 bound drug (nM)arrow_forwardUsing diethylmalonate and benzyl bromide as your only as your only source of carbon, propose a synthesis for the following compound.arrow_forwardplease helparrow_forward
- What is the difference between (+)-(S)-methamphetamine and (-)-(R)-methamphetamine versus levo-methamphetamine and dextro-methamphetamine, D-methamphetamine, and L-methamphetamine, and N-methamphetamine? Please use scholarly sources and in-text citations.arrow_forwardanswer all the questions with explanationarrow_forwardPlease draw a mechanism don't write sentarrow_forward
- From this COZY spectrum, how do you know which protons are next to each other?arrow_forward5. A buffer consists of 0.45 M NH, and 0.25 M NH-CI (PK of NH 474) Calculate the pH of the butter. Ans: 9.52 BAS PH-9.26 +10g (10.95)) 14-4.59 PH=4.52 6. To 500 ml of the buffer on #5 a 0.20 g of sample of NaOH was added a Write the net ionic equation for the reaction which occurs b. Should the pH of the solution increase or decrease sightly? Calculate the pH of the buffer after the addition Ans: 9.54arrow_forwardExplain the inductive effect (+I and -I) in benzene derivatives.arrow_forward
- The inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?arrow_forward19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





