
(a)
Interpretation:
The formula of ionic compound calcium iodide needs to be determined.
Concept introduction:
Ionic bonds are formed when there is complete transfer of electrons from one atom to another. These atoms either lose or gain electrons to become negatively or positively charged ions. The forces of attraction between these ions cause the ionic bond formation. Ionic compounds are formed only when there is a balance between their charges.
(a)
Answer to Problem 81A
The chemical formula for ionic compound calcium iodide is
Explanation of Solution
The element Calcium belongs to Group 2 and has
The element Iodine belongs to Halogen group or group 17 having atomic number as 9. The electronic configuration is
To combine
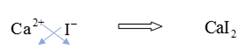
Hence, the formula of the compound calcium iodide is
(b)
Interpretation:
The formula of ionic compound silver bromide needs to be determined.
Concept introduction:
Ionic bonds are formed when there is complete transfer of electrons from one atom to another. These atoms either lose or gain electrons to become negatively or positively charged ions. The forces of attraction between these ions cause the ionic bond formation. Ionic compounds are formed only when there is a balance between their charges.
(b)
Answer to Problem 81A
The chemical formula for ionic compound Silver Bromide is
Explanation of Solution
The element Silver belongs to Group 11 and has atomic number of 47. Its electronic configuration is
The element Bromide belongs to Halogen group or group 17 having atomic number as 35. The electronic configuration is
To combine
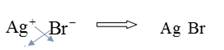
Hence, the formula of the compound Silver Bromide is
(c)
Interpretation:
The formula of ionic compound copper chloride needs to be determined.
Concept introduction:
Ionic bonds are formed when there is complete transfer of electrons from one atom to another. These atoms either lose or gain electrons to become negatively or positively charged ions. The forces of attraction between these ions causes the ionic bond formation. Ionic compounds are formed only when there is a balance between their charges.
(c)
Answer to Problem 81A
The chemical formula for ionic compound Copper chloride is
Explanation of Solution
The element Copper belongs to Group 11 and has atomic number of 29. Its electronic configuration is
The element Chlorine belongs to Halogen group or group 17 having atomic number as 17. The electronic configuration is
To combine

Hence, the formula of the compound Copper chloride is
(d)
Interpretation:
The formula of ionic compound potassium periodate needs to be determined.
Concept introduction:
Ionic bonds are formed when there is complete transfer of electrons from one atom to another. These atoms either lose or gain electrons to become negatively or positively charged ions. The forces of attraction between these ions cause the ionic bond formation. Ionic compounds are formed only when there is a balance between their charges.
(d)
Answer to Problem 81A
The chemical formula for ionic compound potassium periodate is
Explanation of Solution
The element Potassium belongs to Group 1 and has atomic number of 19. Its electronic configuration is
The element Iodine belongs to Halogen group or group 17 having atomic number as 9. The electronic configuration is
Periodate is oxianion of Iodine and is denoted as
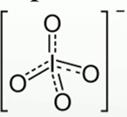
To combine
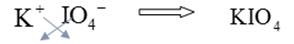
Hence, the formula of the compound potassium periodate is
(e)
Interpretation:
The formula of ionic compound Silver acetate needs to be determined.
Concept introduction:
Ionic bonds are formed when there is complete transfer of electrons from one atom to another. These atoms either lose or gain electrons to become negatively or positively charged ions. The forces of attraction between these ions cause the ionic bond formation. Ionic compounds are formed only when there is a balance between their charges.
(e)
Answer to Problem 81A
The chemical formula for ionic compound Silver acetate is
Explanation of Solution
The element Silver belongs to Group 11 and has atomic number of 47. Its electronic configuration is
The acetate ion is conjugate base of acetic acid. It is depicted as
To combine
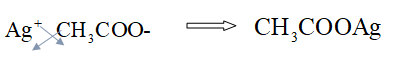
Hence, the formula of the compound Silver acetate is
Chapter 7 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: The Central Science (13th Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (6th Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





