
Interpretation:
The formation of ionic compound from elements Lithium and oxygen needs to be explained.
Concept introduction:
Chemical compounds are formed when the atoms, ions or molecules of two substances are attracted towards each other forming a
Answer to Problem 8PP
2 Lithium atoms will lose 2 electrons which is gained by Oxygen to form ionic compound.
Explanation of Solution
The formation of Lithium oxide takes place when Lithium atoms interact with oxygen atom.
The electronic configuration of Lithium is 1s2 2s1 or [He]2s1
Lithium atom has one outermost electron which can be lost to become a positive ion.
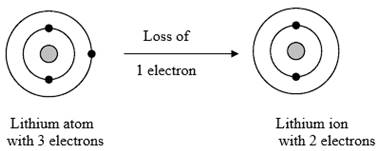
Figure 1
The electronic configuration of Oxygen is 1s2 2s2 2px2 2py1 2pz1
Oxygen atom has 2 half filled shells of p orbital. To fill up the half filled shells, it can take up 2 electrons to become a oxide anion.
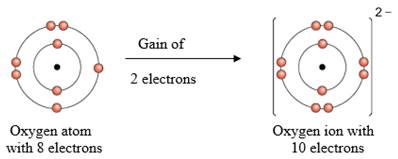
Figure 2
The 2 electrons can be supplied from 2 of Lithium atoms.
Hence, we can say that 2 Lithium atoms will lose 2 electrons that is gained by Oxygen to get an octet structure and form ionic compound.
The overall compound formed is Li2O
2 Lithium atoms will lose 2 electrons which is gained by Oxygen to form ionic compound, Li2O.
Chapter 7 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
The Cosmic Perspective (8th Edition)
Introductory Chemistry (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Consider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forwardPlease help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forward
- Which of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





