
Concept explainers
(a)
Interpretation:
The name of the following molecule should be determined.
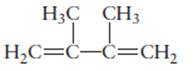
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons.
Rules of naming
- First choose the longest continuous chain of carbon atoms having double bond known as parent chain.
- The numbering of parent chain should be done in a way that the double bond and substituents get the lowest number.
- The root name of the carbon chain is same as in case of
alkanes , but “−ane” ending is replaced by “−ene” - The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The name of the following molecule should be determined.
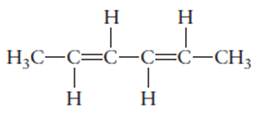
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Unsaturated hydrocarbon having double bond is known as alkene having general molecular formula
Rules of naming alkenes are:
- First choose the longest continuous chain of carbon atoms having double bond known as parent chain.
- The numbering of parent chain should be done in a way that the double bond and substituents get the lowest number.
- The root name of the carbon chain is same as in case of alkanes, but “−ane” ending is replaced by “−ene”
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(c)
Interpretation:
The name of the following molecule should be determined.
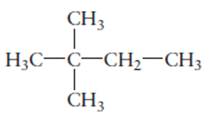
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First choose the longest continuous chain of carbon atoms known as parent chain and determines the base name of alkane.
- The numbering of parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The name of the following molecule should be determined.
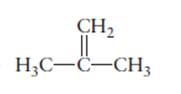
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Unsaturated hydrocarbon having double bond is known as alkene having general molecular formula
Rules of naming alkenes are:
- First choose the longest continuous chain of carbon atoms having double bond known as parent chain.
- The numbering of parent chain should be done in a way that the double bond and substituents get the lowest number.
- The root name of the carbon chain is same as in case of alkanes, but “−ane” ending is replaced by “−ene”
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Principles of Modern Chemistry
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

